Method for preparing lithium iron phosphate cathode material by three-stage high-temperature solid phase calcination
A positive electrode material, lithium iron phosphate technology, applied in chemical instruments and methods, phosphorus compounds, battery electrodes, etc., can solve problems such as high price, cycle life, poor thermal stability and high temperature performance, and potential safety hazards, and achieve safety And the effect of good stability, superior electrochemical performance, easy control and operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
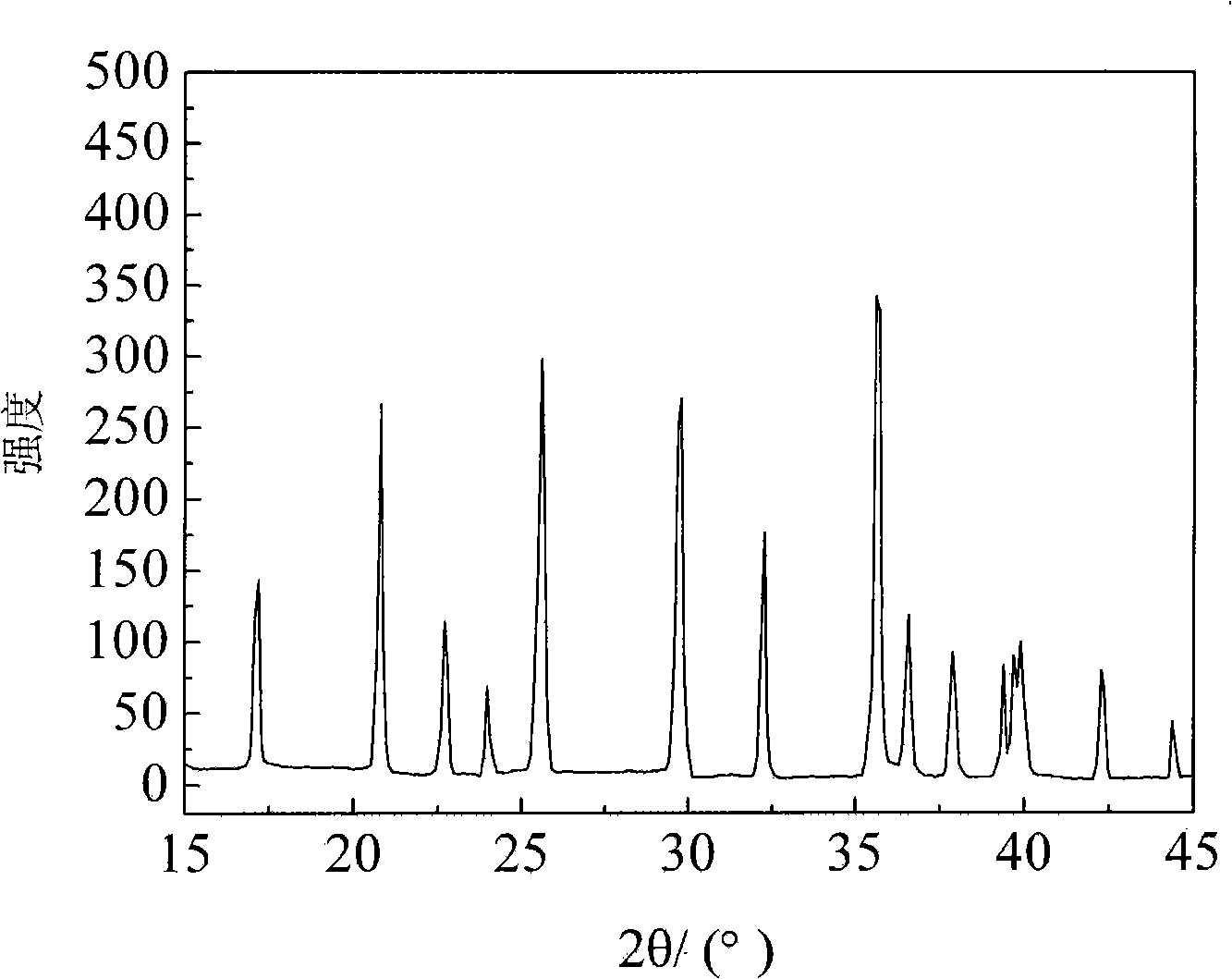
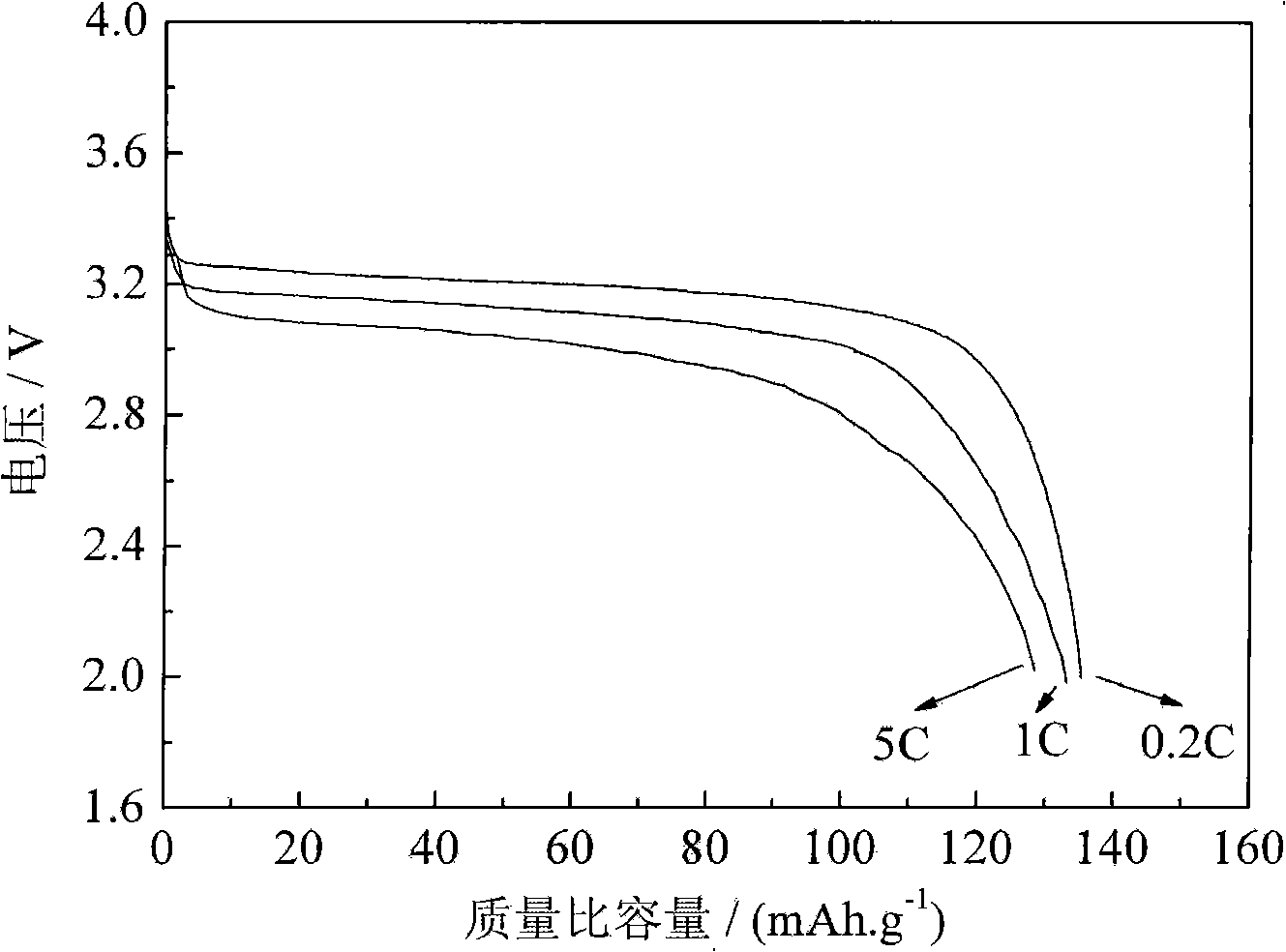
[0019] Will Li 2 CO 3 , FeC 2 o 4 2H 2 O and NH 4 h 2 PO 4 Weighing according to the stoichiometric ratio of 0.5:1:1, a total of 332 grams (of which Li 2 CO 3 : 37 g; FeC 2 o 4 2H 2 O: 180 g; NH 4 h 2 PO 4 : 115 grams), and add 13 grams of glucose relative to the raw material weight 4%, grind 5h in the wet method agitation type ball mill. Dry and granulate the suspension obtained above, transfer the obtained spherical particles to a rotary sintering furnace, raise the temperature to 300°C for pre-decomposition for 5 hours under a nitrogen protective atmosphere, then raise the temperature to 750°C, keep it warm for 12 hours, and then drop to room temperature with the furnace temperature , the obtained sample was added with 2% glucose relative to the weight of the raw material, and after ball milling for 12 hours, the temperature was raised to 750° C., kept for 6 hours and then lowered to room temperature with the furnace temperature, and the final lithium iron pho...
Embodiment 2
[0023] Will Li 2 CO 3 , FeC 2 o 4 2H 2 O and (NH 4 ) 2 HPO 4 Weighing according to the stoichiometric ratio of 0.5:1:1, a total of 332 grams (of which Li 2 CO 3 : 37 g; FeC 2 o 4 2H 2 O: 180 g; NH 4 h 2 PO 4 : 115 grams), and add 6.5 grams of glucose relative to raw material weight 2%, grind 5h in the wet-type stirring ball mill. Dry and granulate the suspension obtained above, transfer the obtained spherical particles to a rotary sintering furnace, raise the temperature to 300°C for pre-decomposition for 5 hours under a nitrogen protective atmosphere, then raise the temperature to 700°C, keep it warm for 12 hours, and then drop to room temperature with the furnace temperature 13 grams of glucose 4% relative to the weight of the raw material was added to the obtained sample, and after ball milling for 12 hours, the temperature was raised to 700°C, kept for 6 hours and then lowered to room temperature with the furnace temperature, and the final lithium iron phosph...
Embodiment 3
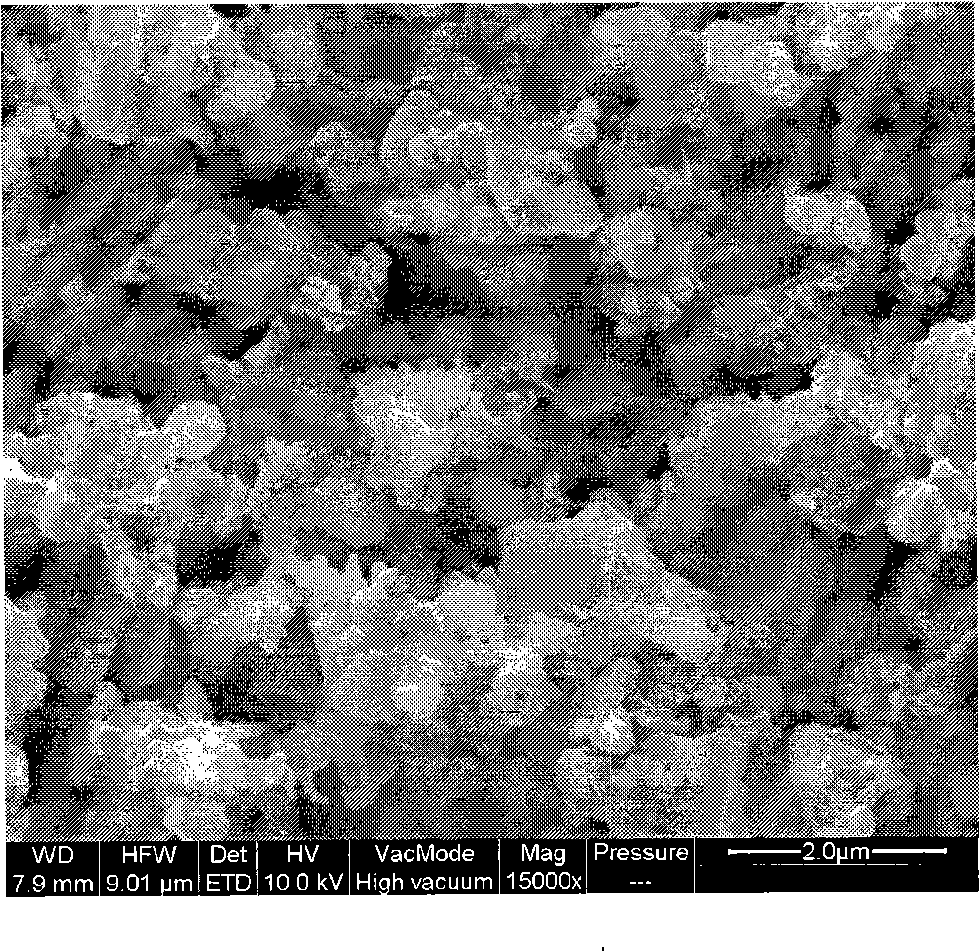
[0026] LiOH·H 2 O, FeC 2 o 4 2H 2 O and (NH 4 ) 2 HPO 4 Weighing according to the stoichiometric ratio of 1:1:1, a total of 337 grams (of which LiOH·H 2 O: 42 g; FeC 2 o 4 2H 2 O: 180 g; NH 4 h 2 PO 4 : 115 grams), ground 5h in a wet stirring ball mill. Dry the above-obtained suspension and granulate it. Transfer the obtained spherical particles to a rotary sintering furnace, raise the temperature to 300°C for pre-decomposition for 5 hours under a nitrogen protective atmosphere, then raise the temperature to 650°C, keep it warm for 12 hours, and then drop to room temperature with the furnace temperature , add 20 grams of glucose 6% relative to the weight of the raw material to the obtained sample, after ball milling for 12 hours, then raise the temperature to 650°C, keep warm for 6 hours and then drop to room temperature with the furnace temperature, and get the final lithium iron phosphate-carbon coating material after ball milling for 12 hours . The average par...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com