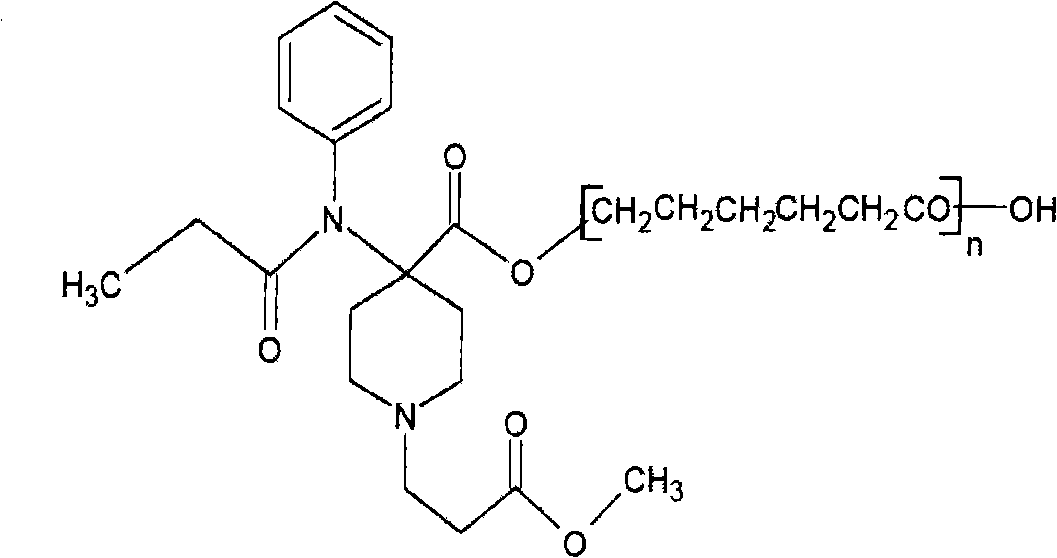
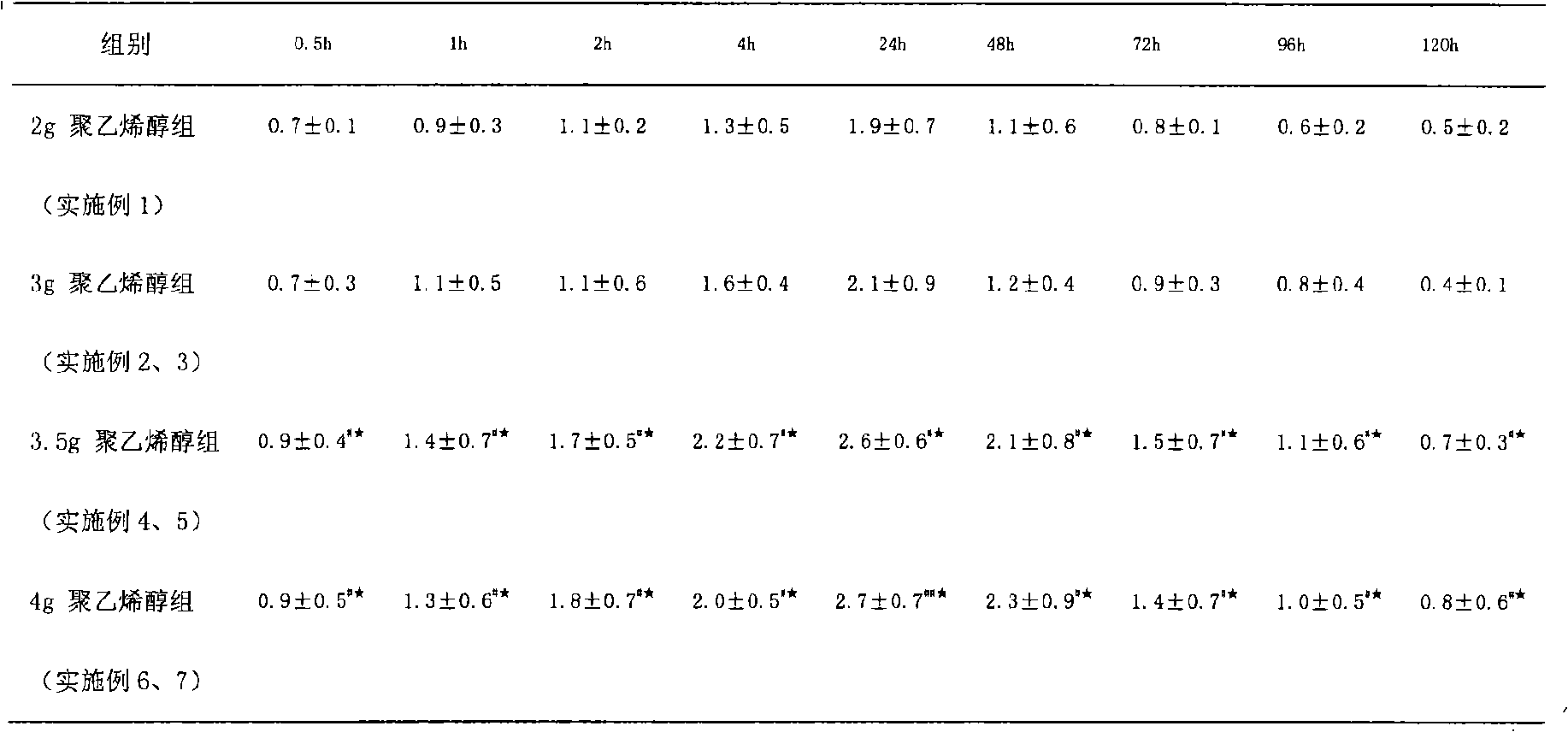Sustained-release analgesia medical dressing and preparation method thereof
A slow-release, medical technology, applied in the field of medicine, can solve the problem of difficult control of the dosage, and achieve the effects of no sensitization, easy industrial production, and simple preparation method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] 1. The first preparation method
[0051] (1) Preparation of remifentanil polymer sustained-release analgesic drug
[0052] A. Raw materials
[0053] 6 grams of caprolactone, 0.008 grams of remifentanil (REM), 6 grams of toluene, 0.006 grams of aluminum isopropoxide;
[0054] B. Polymerization reaction
[0055] First put toluene into a reaction kettle with a stirrer, then add caprolactone and aluminum isopropoxide to toluene, under the protection of argon, react at normal pressure and 30°C for 40 hours, then add REM, , 30 ° C for 4 hours, the whole reaction is carried out under stirring;
[0056] C. Collect the product from the reaction mixture
[0057] After the polymerization reaction is completed, remove the solvent by distillation under reduced pressure, cool to room temperature, and then dissolve the product with chloroform, then add cold methanol at 4°C for precipitation (the number of precipitation is at least 3 times), and the white product obtained after suc...
Embodiment 2
[0078] 1. The first preparation method
[0079] (1) Preparation of remifentanil polymer sustained-release analgesic drug
[0080] A. Raw materials
[0081] 6 grams of caprolactone, 0.008 grams of remifentanil (REM), 9 grams of tetrahydrofuran, 0.4 grams of zinc octanoate;
[0082] B. Polymerization reaction
[0083] First put tetrahydrofuran into a reaction kettle with a stirrer, then add caprolactone and zinc octanoate into tetrahydrofuran, under the protection of helium, react at normal pressure and 130°C for 4 hours, then add REM, ℃ for 1.5 hours, and the whole reaction was carried out under stirring;
[0084] C. Collect the product from the reaction mixture
[0085] After the polymerization reaction is completed, remove the solvent by distillation under reduced pressure, cool to room temperature, and then dissolve the product with chloroform, then add cold methanol at 4°C for precipitation (the number of precipitation is at least 3 times), and the white product obtaine...
Embodiment 3
[0106] 1. The first preparation method
[0107] (1) Preparation of remifentanil polymer sustained-release analgesic drug
[0108] A. Raw materials
[0109] 6 grams of caprolactone, 0.008 grams of remifentanil (REM), 9 grams of toluene, 0.002 grams of iron acetate;
[0110] B. Polymerization reaction
[0111] First put toluene into a reaction kettle with a stirrer, then add caprolactone and iron acetate into toluene, under the protection of argon, react at normal pressure and 120°C for 12 hours, then add REM, ℃ for 2 hours, and the whole reaction was carried out under stirring;
[0112] C. Collect the product from the reaction mixture
[0113] After the polymerization reaction is completed, remove the solvent by distillation under reduced pressure, cool to room temperature, and then dissolve the product with chloroform, then add cold methanol at 4°C for precipitation (the number of precipitation is at least 3 times), and the white product obtained after suction filtration i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| water absorption | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com