Method for synthesizing gallic acid terpene alcohol ester with auxiliary supersonic wave
A technology of terpene gallate and gallic acid, which is applied in the field of synthesizing terpene gallate, can solve the problems of small preparation scale, long reaction time, cumbersome operation, etc., and achieve production cost reduction, simple operation, and simple reaction system Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
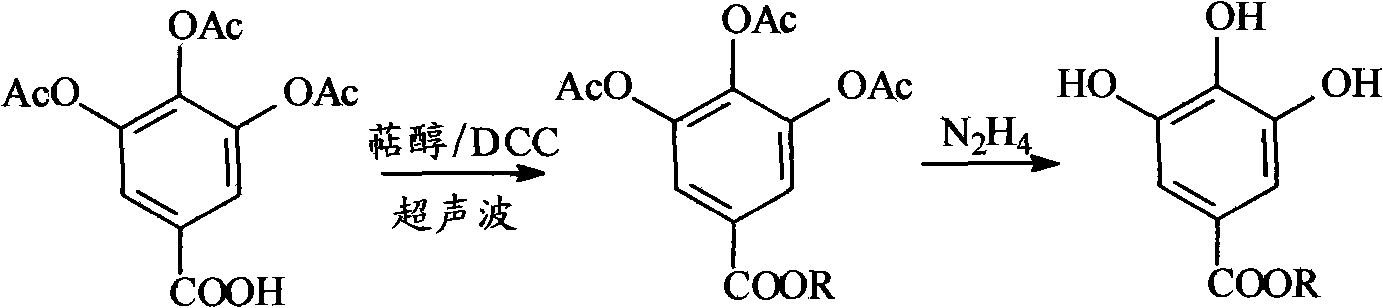
[0025]Mix 500-750 mL of acetic anhydride with 2 mL of sulfuric acid, wherein 2 mL of sulfuric acid can be replaced by 4 g of p-toluenesulfonic acid or 6 g of potassium hydrogensulfate. Then add 170 g of anhydrous gallic acid in batches, after the addition is complete, slowly raise the temperature to 90-110°C, and keep it warm for 1-3 hours. After cooling to room temperature, the mixed product was poured into 500-2000 mL of ice water to produce a white solid, which was filtered, washed with water, and dried to obtain 230-260 g of 3,4,5-triacetyl gallic acid.
Embodiment 2
[0027] Mix 7.4g of 3,4,5-triacetyl gallic acid with farnesol 5.7g / DCC6.3g / acetonitrile 7.4~740g, and ultrasonically react at room temperature for 0.01~3h, remove DCU by filtration to obtain 3,4 , the acetonitrile solution of terpene alcohol 5-triacetyl gallate; to the acetonitrile solution of terpene alcohol triacetyl gallate, add 4.4 g of hydrazine hydrate with a concentration of 85% by mass, and stir at room temperature for 10 to 30 min , add acetic acid in the same amount as hydrazine hydrate and 14.4~370g of water, stir at room temperature for 1~5min, add 50~300mL ethyl acetate for extraction, separate layers, wash the ethyl acetate layer with 100~300mL water, add 5~ 20g of anhydrous sodium sulfate was dried, and the ethyl acetate was distilled off at 40-50°C at -0.09--0.05MPa to obtain 8.8g of farnesyl gallate with a conversion rate of over 90%.
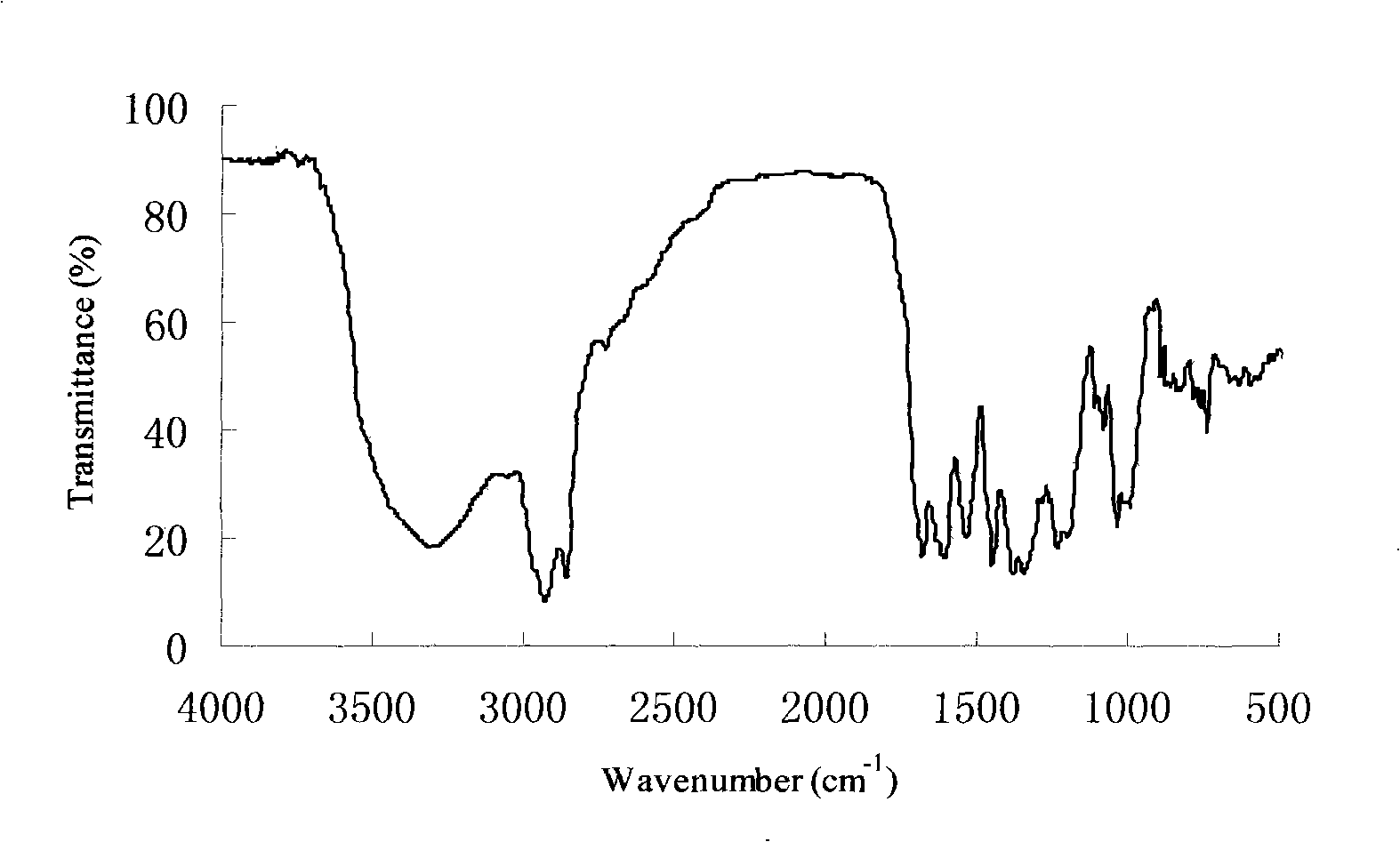
[0028] The infrared spectrogram of farnesyl gallate is shown in the appendix figure 1 . Depend on figure 1 It can be seen t...
Embodiment 3
[0030] Mix 7.4g of 3,4,5-triacetyl gallic acid with 4g of menthol / 6.3g of DCC / 7.4-740g of acetonitrile, ultrasonically react at room temperature for 0.01-3h, and remove DCU by filtration to obtain 3,4,5 -Acetonitrile solution of terpene triacetyl gallate; to the acetonitrile solution of terpene triacetyl gallate, add 4.4g of 85% hydrazine hydrate, stir at room temperature for 10-30min, add hydrazine hydrate, etc. The amount of acetic acid and water 14.4~370g, stirring at room temperature for 1~5min, adding 50~300mL ethyl acetate for extraction, layering, washing the ethyl acetate layer with 100~300mL water, adding 5~20g anhydrous sodium sulfate to dry , 40 ~ 50 ° C, -0.09 ~ -0.05MPa distilled ethyl acetate, to obtain light brown yellow menthyl gallate 7.4g, the conversion rate of more than 90%.
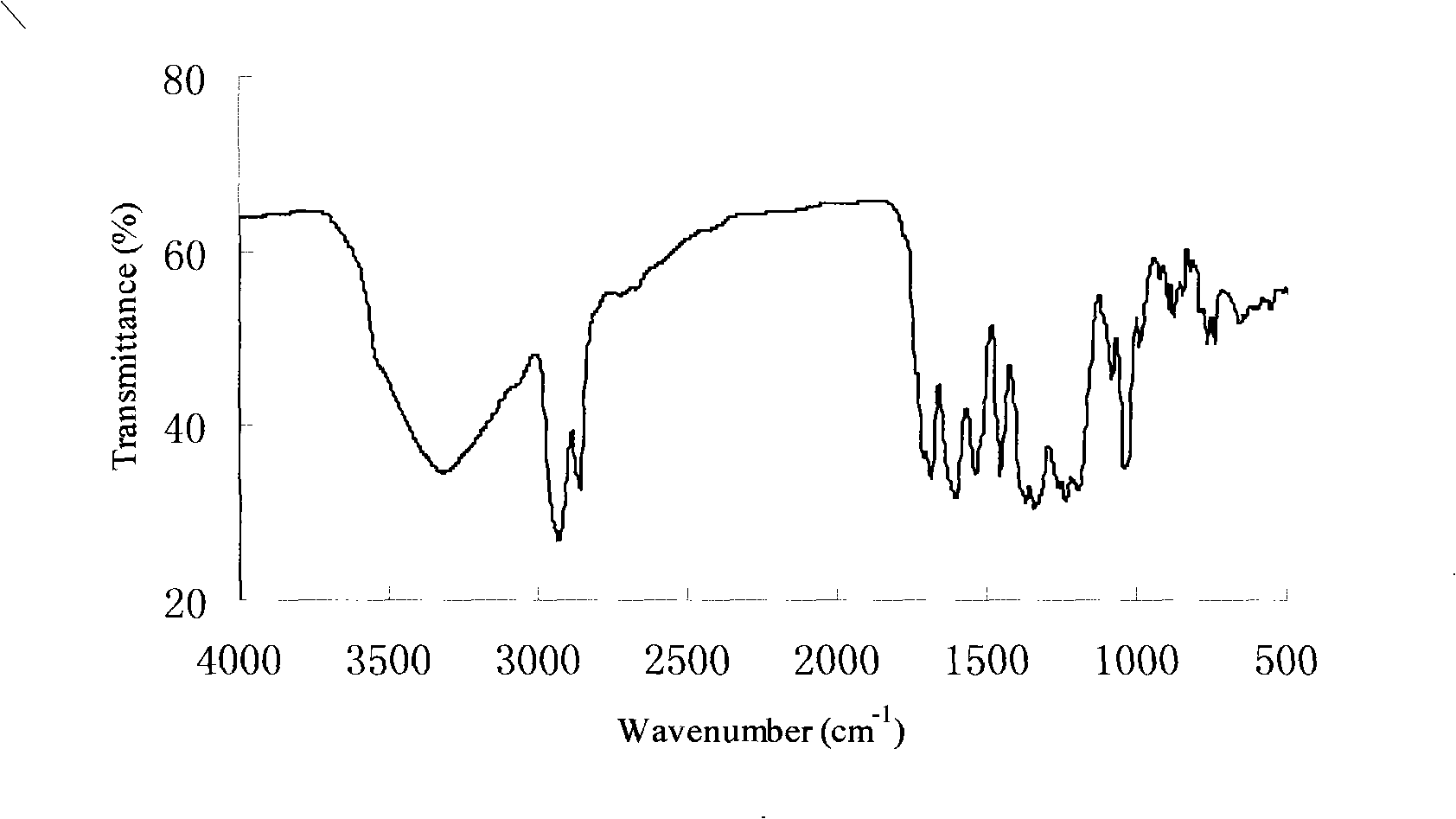
[0031] The infrared spectrogram of menthyl gallate is shown in the appendix figure 2 . Depend on figure 1 It can be seen that 3313cm -1 The nearby strong and broad absorption pea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com