Campylobacter jejuni chitose nano DNA vaccine and preparation method and use thereof
A Campylobacter jejuni, chitosan nanotechnology, applied in recombinant DNA technology, botanical equipment and methods, biochemical equipment and methods, etc., can solve the problems of low antibody titer, weak preventive effect, poor immune effect, etc. To achieve the effect of convenient operation and safe immune process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
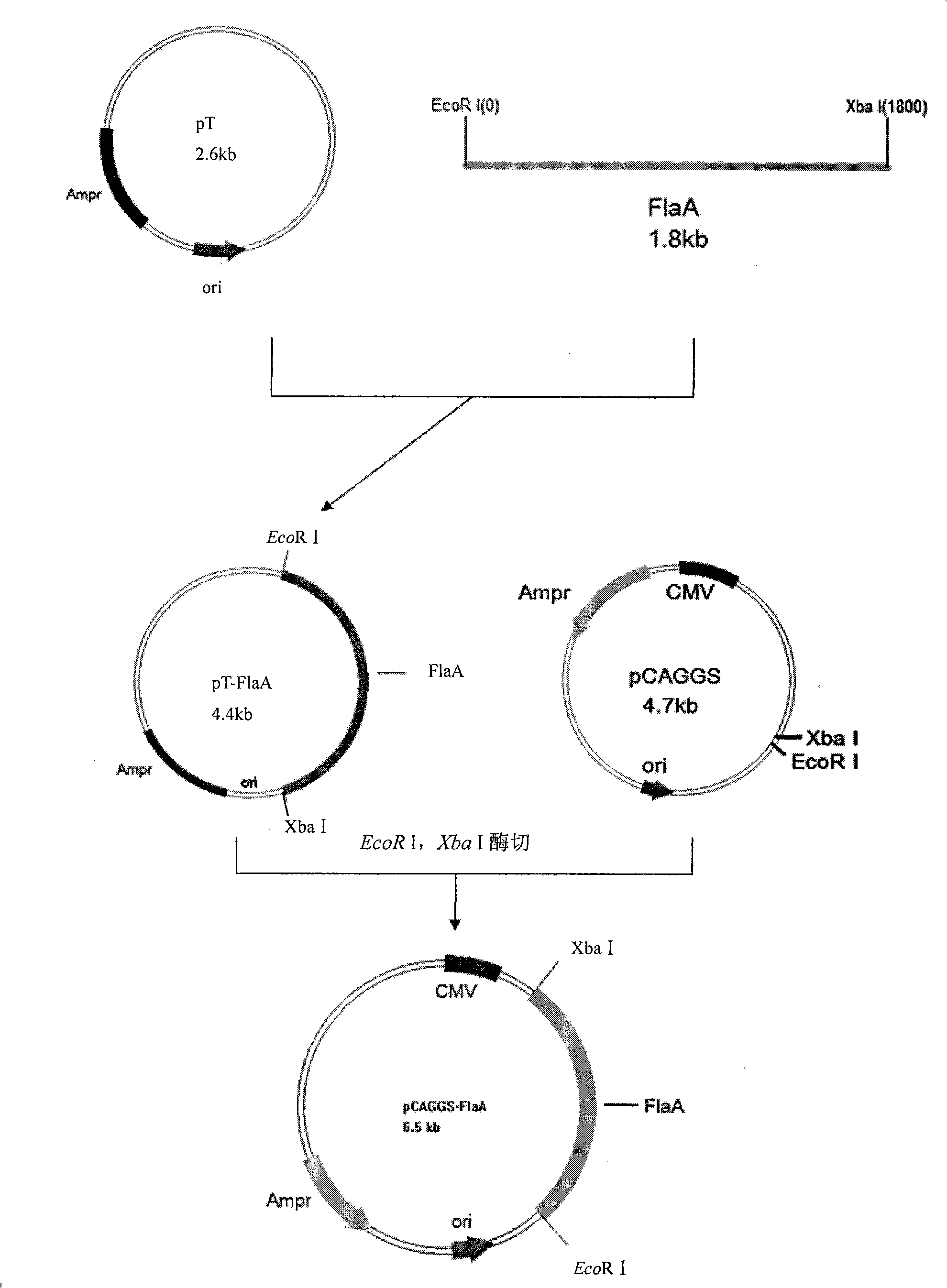
[0032] Embodiment 1. Campylobacter jejuni DNA vaccine of the present invention--the construction of pCAGGS-flaA DNA vaccine
[0033] The construction steps of the pCAGGS-flaA DNA vaccine of the present invention are as follows:
[0034] ① Extraction of Campylobacter jejuni genome template
[0035] Take 0.5ml of the pure culture of Campylobacter jejuni, centrifuge at 8000rpm for 5min, discard the supernatant, add 100μl of ultrapure water, mix it evenly with a pipette, boil in water for 20min, take it out and put it on ice immediately, and centrifuge at 8000rpm for 5min. Take the supernatant and store it in a -20°C refrigerator for later use.
[0036] ② Design specific primers: According to the GenBank entry sequence (gi: 30407139), use DNAstar software to analyze, select the nucleotide sequence of Campylobacter jejuni flagellin gene flaA, and design synthetic primers according to the correct reading order. Restriction sites and protective bases are added. The upstream primer...
Embodiment 2
[0053] Example 2. Identification of transient expression of exogenous flaA gene in vitro by pCAGGS-flaA DNA vaccine
[0054] To establish a transient expression system for COS-7 cells in vitro, the eukaryotic expression plasmid pCAGGS-flaA constructed in Example 1 was prepared in small quantities with QIAfilter plasma midi kit, and transfected into COS-7 cells cultured in 24-well plates via liposomes In order to form DNA-liposome complex; 48h later, the expression of flaA gene was detected by indirect immunofluorescence test. The transfected cells were washed 3 times with PBS, dried naturally, fixed with -20°C pre-cooled anhydrous methanol for 5 min, the fixative was discarded, and washed 3 times with PBS. Add 800 μL 1:400 dilution of chicken anti-Campylobacter jejuni polyantiserum, incubate in 37°C incubator for 60 min, wash with PBS three times, add 800 μL 1:160 dilution of FITC-labeled rabbit anti-chicken fluorescent secondary antibody, 37°C incubator Incubate for 40 min, ...
Embodiment 3
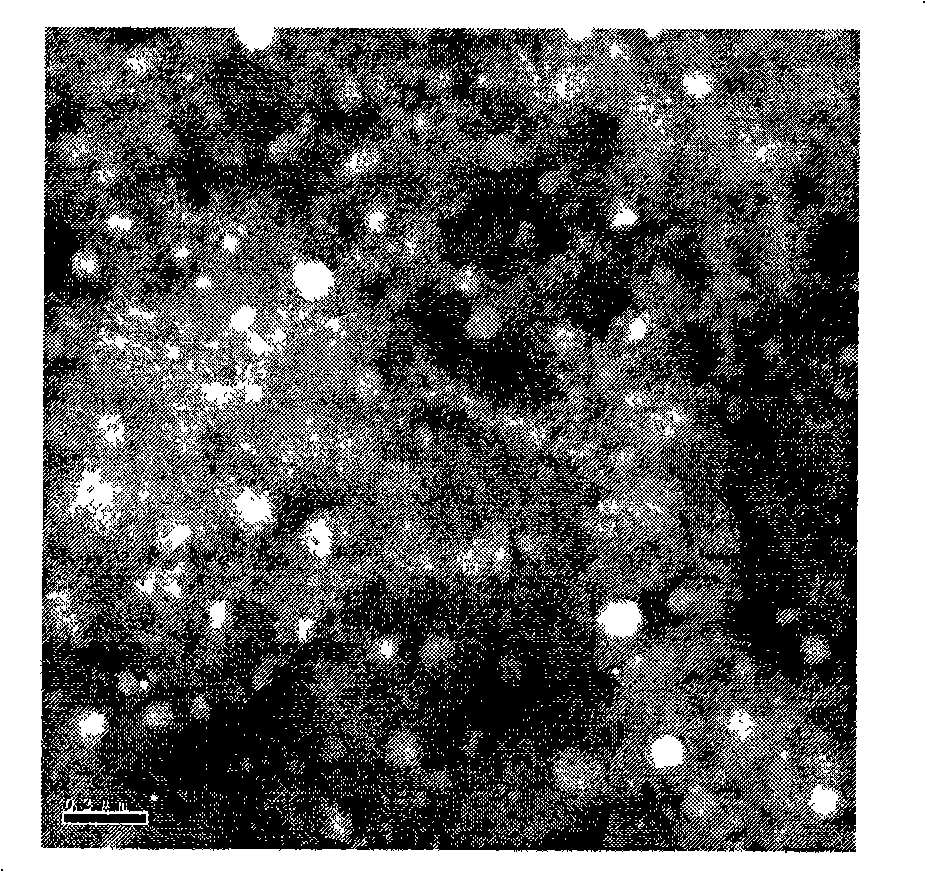
[0055] Embodiment 3. Preparation of chitosan-plasmid nanoparticles
[0056] Chitosan (Chitosan, hereinafter referred to as CS) was purchased from Zhejiang Jinke Biochemical Co., Ltd., batch number D070831151, yellow powder, molecular weight 201Kda, deacetylation degree 87%, viscosity 20mpa.s. Weigh 200mg of chitosan and add 800μl of acetic acid, then add SW to 5mL, shake overnight at 37°C 180r / min to completely dissolve chitosan (purchased from Zhejiang Jinke Biochemical Co., Ltd.), add water to 480mL the next day and adjust with NaOH The pH is 5.5, and the final volume is 500mL, and vacuum filtration (0.22μm membrane) is sterilized to obtain liquid a.
[0057] Dissolve the constructed pCAGGS-flaADNA vaccine in sterile 5mmoL / L Na 2 SO4 solution to 300 μg / mL to obtain liquid b.
[0058] Heat equal amounts of liquid a and liquid b to 55°C for 10-15 minutes respectively, mix evenly at a ratio of 1:1, shake for 30 seconds to obtain chitosan-coated plasmid DNA immune microcapsule...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com