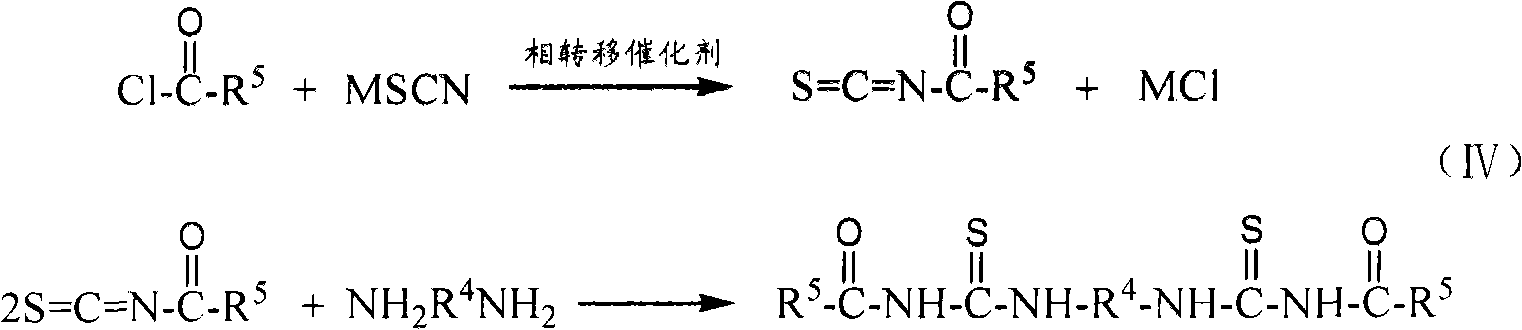
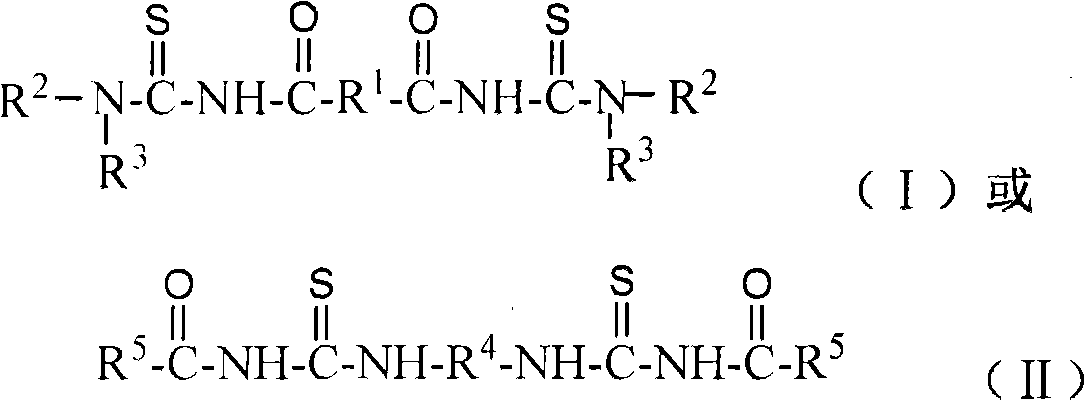Sulphide ore floation collector and use method of diacyl bis-thiourea and preparation method thereof
A diacylbisthiourea, application method technology, applied in flotation, organic chemistry, solid separation and other directions, can solve the problems of low comprehensive utilization level and poor selectivity of sulfide ore, achieve good selectivity, simple preparation process, strong The effect of harvesting power
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
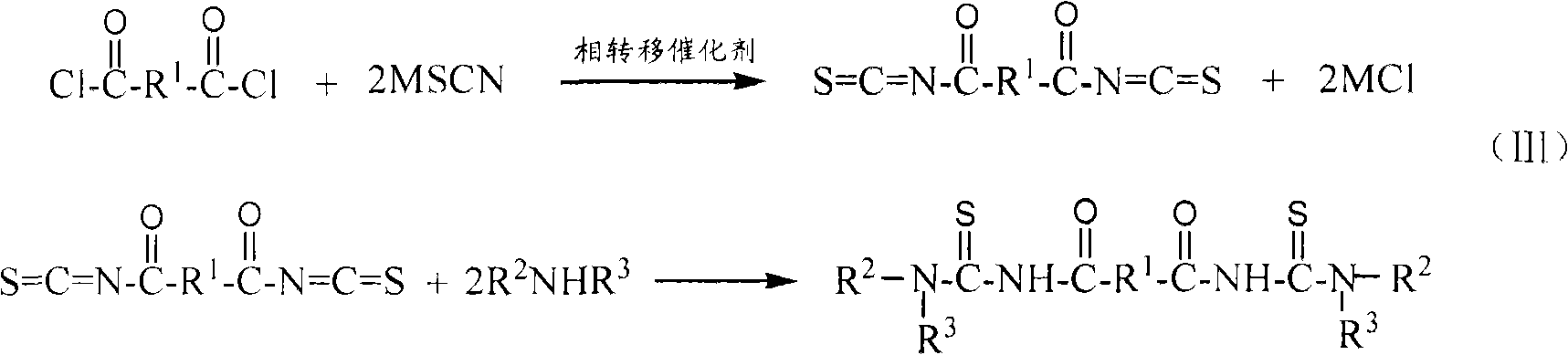
[0036] Example 1: Synthesis of N,N'-dipropyl-N",N"'-(terephthaloyl)bis(thiourea)
[0037] In a 250ml three-necked flask, add 2.03g terephthaloyl chloride (0.01mol), 3 drops of PEG-400 (about 0.2g), 2.46g potassium thiocyanate (0.025mol) and 40ml dichloromethane, and stir at 20℃ The reaction was carried out for 3.5 hours to obtain a yellow reaction liquid containing the terephthaloyl diisothiocyanate intermediate. Then, at 20°C, slowly drop 10ml of dichloromethane solution in which 1.68ml of n-propylamine (0.02mol) is dissolved into the above three-necked flask containing the terephthaloyl diisothiocyanate intermediate, and continue after dropping. The reaction was carried out at 20°C for 1 hour to obtain a reaction liquid containing N,N'-dipropyl-N",N"'-(terephthaloyl)bis(thiourea). The reaction solution was suction filtered to remove the by-product potassium chloride, and the dichloromethane was evaporated under reduced pressure to obtain N,N'-dipropyl-N",N"'-(terephthaloyl)bis(t...
Embodiment 2
[0038] Example 2: Synthesis of N,N'-Dibutyl-N",N"'-(terephthaloyl)bis(thiourea)
[0039] In a 250ml three-necked flask, add 2.03g terephthaloyl chloride (0.01mol), 3 drops of PEG-400 (about 0.2g), 2.46g potassium thiocyanate (0.025mol) and 40ml dichloromethane, stir at 15℃ The reaction was carried out for 3.5 hours to obtain a yellow reaction liquid containing the terephthaloyl diisothiocyanate intermediate. Then, at 15°C, slowly drop 10ml of methylene chloride solution in which 2.01ml of n-propylamine (0.02mol) is dissolved into the above three-necked flask containing terephthaloyl diisothiocyanate intermediate, and continue after dropping. The reaction was carried out at 15°C for 1 hour to obtain a reaction liquid containing N,N'-dibutyl-N",N"'-(terephthaloyl)bis(thiourea). The reaction solution was suction filtered to remove the by-product potassium chloride, and the dichloromethane was evaporated under reduced pressure to obtain N,N'-dibutyl-N",N"'-(terephthaloyl)bis(thiourea)...
Embodiment 3
[0040] Example 3: Synthesis of N,N'-Dipropyl-N",N"'-(1,4-succinyl)bis(thiourea)
[0041] In a 250ml three-necked flask, add 1.55g 1,4-succinyl chloride (0.01mol), 3 drops of PEG-400 (about 0.2g), 2.46g potassium thiocyanate (0.025mol) and 40ml dichloromethane at 20℃. The reaction was stirred for 3.5 hours to obtain a reaction liquid containing 1,4-succinyl diisothiocyanate intermediate. Then, at 20°C, slowly drop 10ml of dichloromethane solution in which 1.68ml of n-propylamine (0.02mol) is dissolved into the above three-necked flask containing 1,4-succinoyl diisothiocyanate intermediate, and add dropwise After continuing the reaction at 20°C for 1 hour, a reaction solution containing N,N'-dipropyl-N",N"'-(1,4-succinyl)bis(thiourea) was obtained. The reaction liquid was suction filtered to remove the by-product potassium chloride, and the dichloromethane was evaporated under reduced pressure to obtain N,N'-dipropyl-N",N"'-(1,4-succinyl)bis( As the initial product of thiourea, the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com