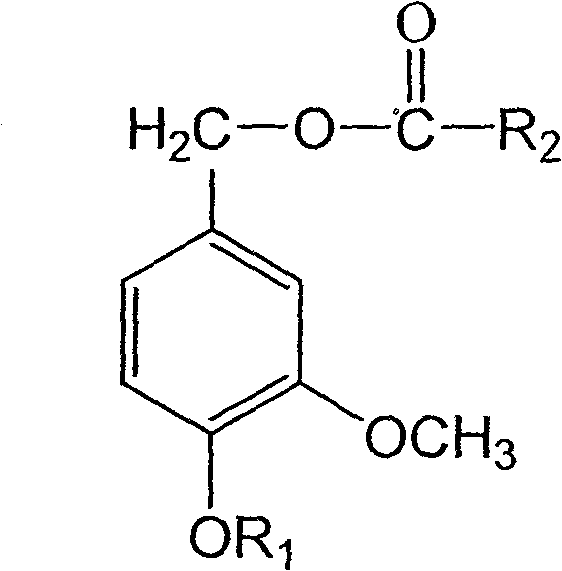
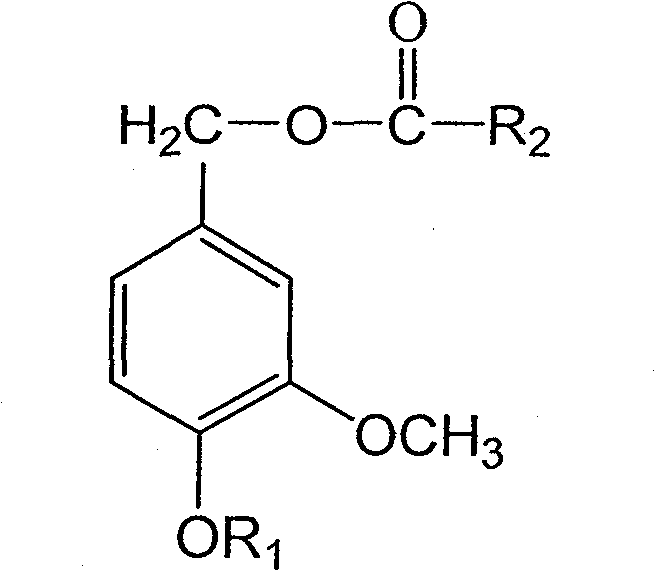
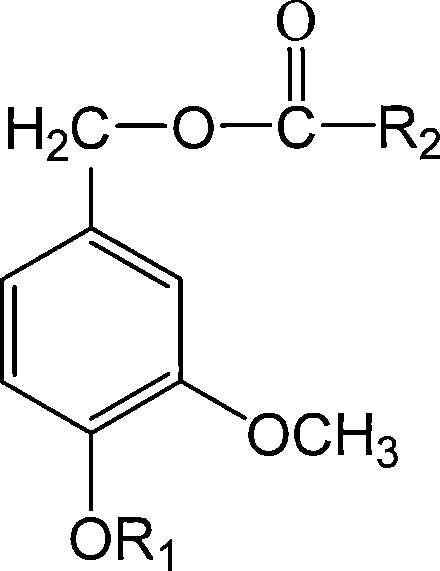
4-alkoxy-coumarin fatty acid ester, synthetic method and use
A technology of fatty acid ester and synthesis method, which is applied in the direction of drug combination, carboxylic acid halide preparation, metabolic diseases, etc., to achieve safe analgesia and weight loss drugs, and less stimulating effects such as spicy taste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1: Preparation of 4-ethoxyl-coumarin caprate
[0059] ①. Under nitrogen protection, dehydrated dimethyl sulfoxide (DMSO) 5.0ml, Cs 2 CO 3 (0.1mmol), K 2 CO 3 (2.0mmol), vanillyl alcohol (1.0mmol) and ethyl bromide (1.2mmol) were sequentially added into a round bottom flask, and the reaction was stirred at room temperature for 15h.
[0060] ② After the reaction is completed, add 100ml of isopropyl ether to dilute, then filter the contents of the flask, wash with water, and wash with anhydrous Na 2 SO 4 dry. After the solvent was removed, it was purified by silica gel column chromatography (petroleum ether / ethyl acetate=5:2) to obtain a colorless oily liquid, which was the 4-ethoxy-coumarin intermediate.
[0061] ③. Under nitrogen protection, dissolve 0.5mmol of 4-ethoxy-coumarin intermediate in 8ml of anhydrous tetrahydrofuran, then add 0.5mmol of decanoyl chloride, 0.081mmol of SbCl 3 , Stir the reaction at room temperature for 12h.
[0062] ④, after ...
Embodiment 2
[0064] Embodiment 2: Preparation of 4-octanyloxy-coumarin caprate
[0065] ①. Under nitrogen protection, dehydrated dimethyl sulfoxide (DMSO) 5.0ml, Cs 2 CO 3 (0.1mmol), K 2 CO 3 (2.0mmol), vanillyl alcohol (1.0mmol) and bromooctane (1.2mmol) were sequentially added into a round bottom flask, and the reaction was stirred at room temperature for 15h.
[0066] ② After the reaction is completed, add 100ml of isopropyl ether to dilute, then filter the contents of the flask, wash with water, and wash with anhydrous Na 2 SO 4 dry. After the solvent was removed, it was purified by silica gel column chromatography (petroleum ether / ethyl acetate=5:2) to obtain a colorless oily liquid, namely 4-octanyloxy-coumarin intermediate.
[0067] ③. Under nitrogen protection, dissolve 0.5mmol of 4-octyloxy-coumarin intermediate in 8ml of anhydrous tetrahydrofuran, then add 0.5mmol of decanoyl chloride, 0.081mmol of SbCl 3 , Stir the reaction at room temperature for 12h.
[0068] ④, after ...
Embodiment 3
[0070] Embodiment 3: Preparation of 4-tetradecyloxy-coumarin caprate
[0071] ①. Under nitrogen protection, dehydrated dimethyl sulfoxide (DMSO) 5.0ml, Cs 2 CO 3 (0.1mmol), K 2 CO 3 (2.0mmol), vanillyl alcohol (1.0mmol) and tetradecane bromide (1.2mmol) were sequentially added into a round bottom flask, and the reaction was stirred at room temperature for 15h.
[0072] ② After the reaction is completed, add 100ml of isopropyl ether to dilute, then filter the contents of the flask, wash with water, and wash with anhydrous Na 2 SO 4 dry. After the solvent was removed, it was purified by silica gel column chromatography (petroleum ether / ethyl acetate=5:2) to obtain a colorless oily liquid, which was the 4-tetradecyloxy-coumarin intermediate.
[0073] ③. Under nitrogen protection, dissolve 0.5mmol of 4-tetradecyloxy-coumarin intermediate in 8ml of anhydrous tetrahydrofuran, then add 0.5mmol of decanoyl chloride, 0.081mmol of SbCl3 , Stir the reaction at room temperature for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com