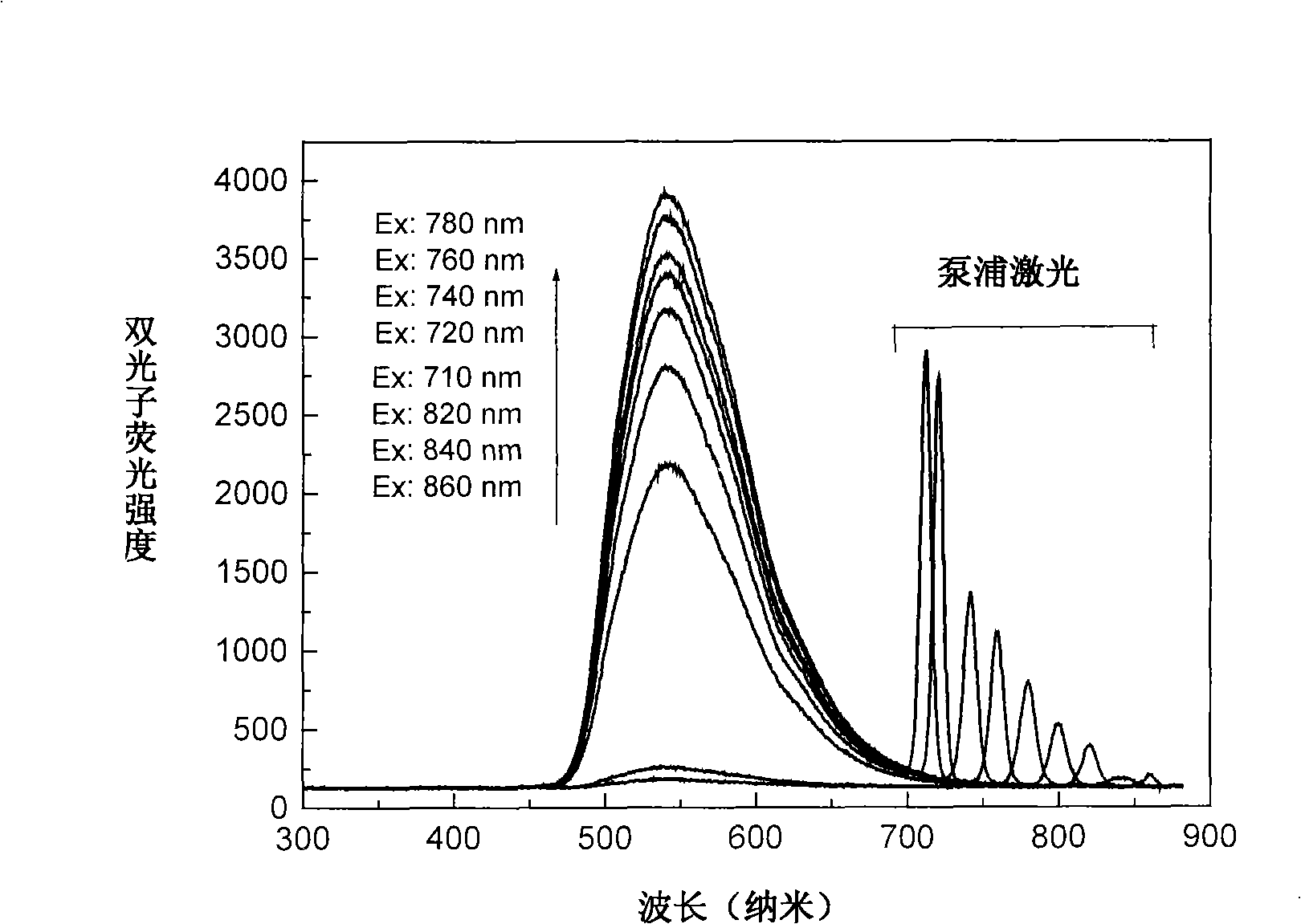
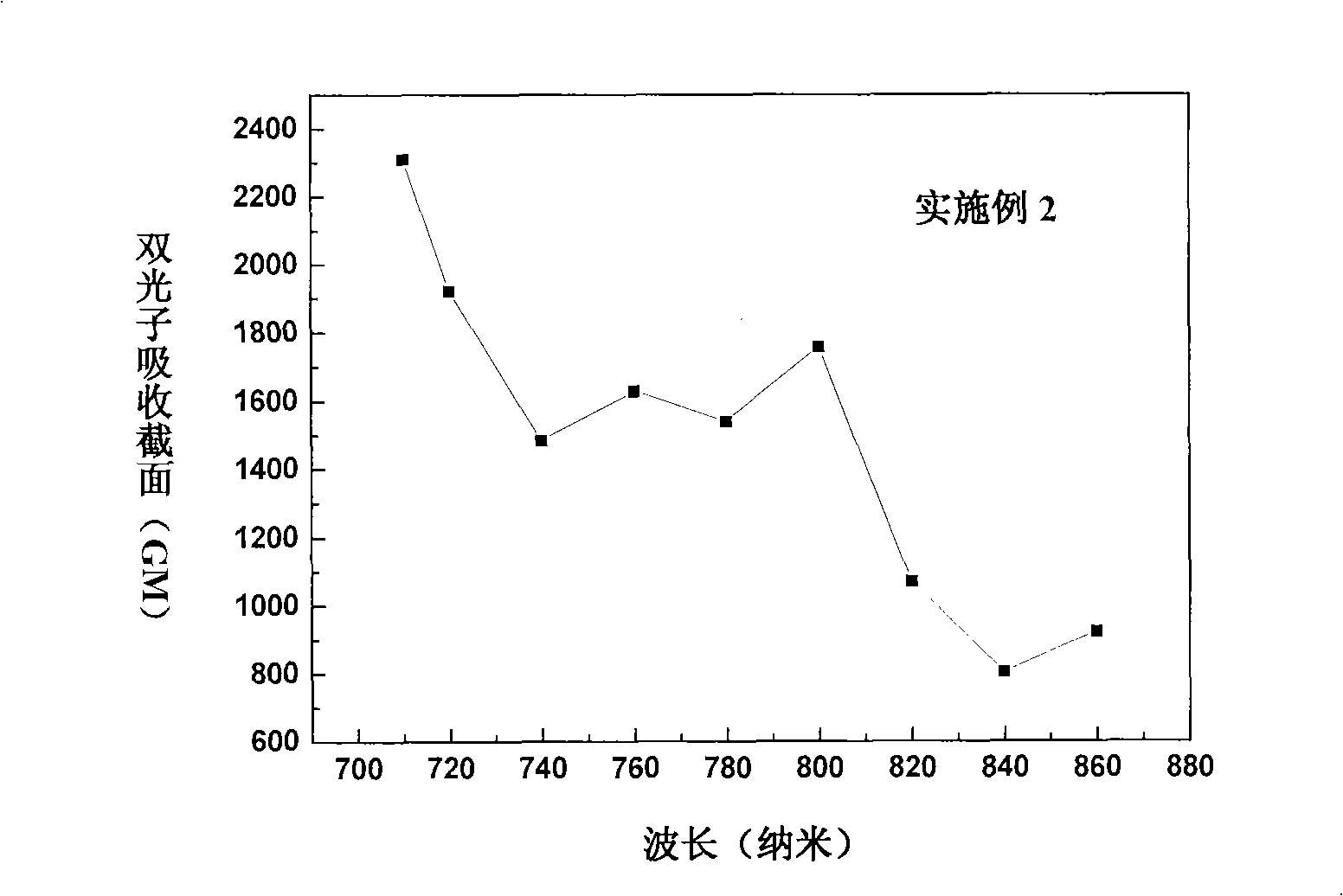
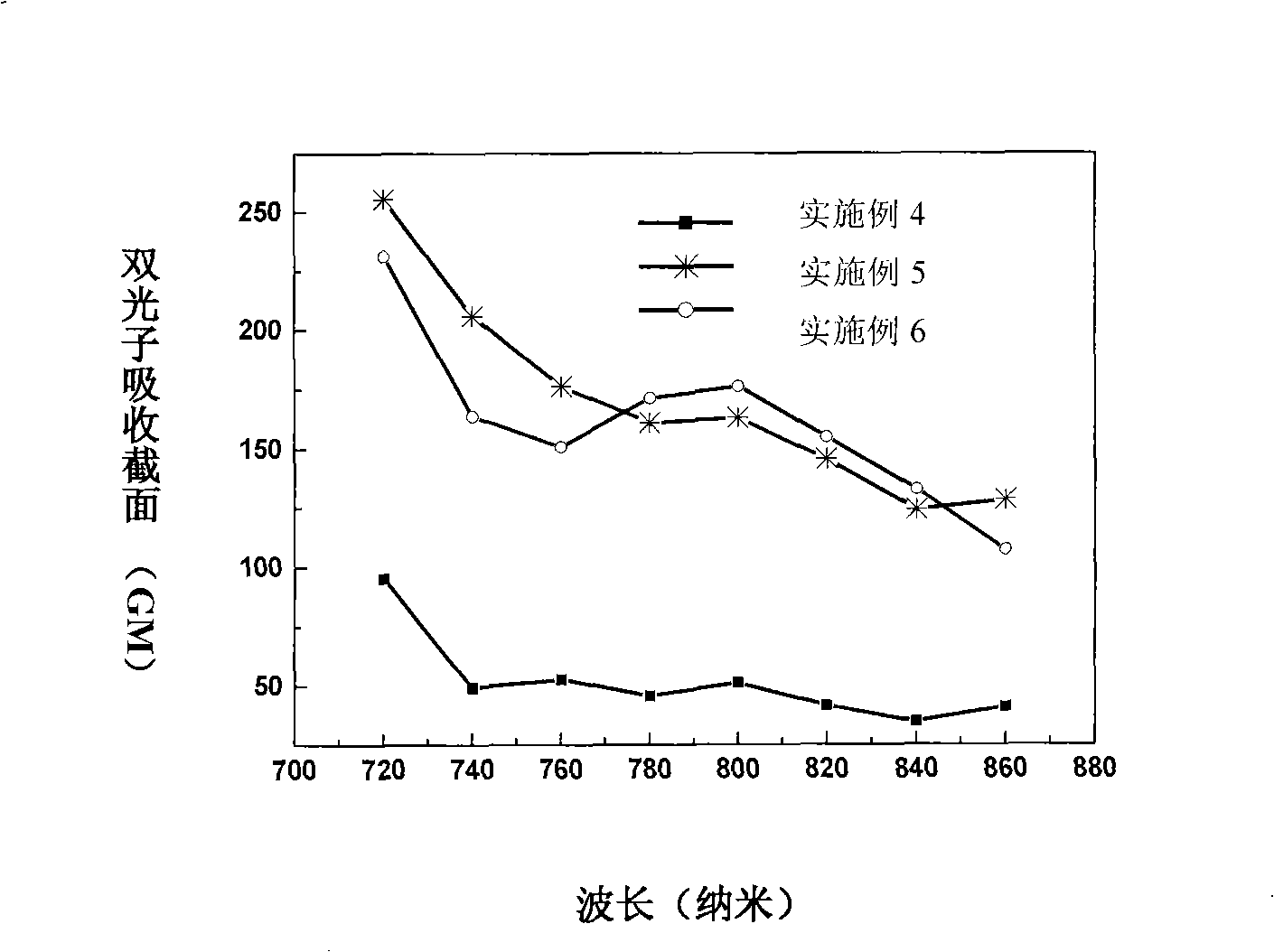
Triphenyl amine-naphthalin multi-branched molecule having two-photon polymerization initiation characteristic
A two-photon polymerization and triphenylamine technology, applied in the field of triphenylamine-naphthalene multi-branch molecules, can solve the problems of low polymerization efficiency, small two-photon absorption cross-section, low photosensitivity, etc. The effect of high microstructure fineness and low aggregation threshold
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Synthesis of 4,4',4"-tris(4-bromo-1-naphthylvinyl)triphenylamine.
[0032] (1) Preparation of 4,4',4"-triphenylamine trialdehyde: under a nitrogen atmosphere, first place 2.4g (0.62mol) of 4,4',4"-triphenylamine tribromide in a three-necked flask, seal the After degassing, 30 mL of dry THF was injected, 3.5 mL of dry DMF was injected at -78°C, and 15.6 mL of n-butyllithium was injected dropwise with stirring. After dropping, react at -78°C for 3 hours, then react at room temperature for 3 hours, stop the reaction, and pour the reaction solution into NH 4 Cl saturated aqueous solution, CH 2 Cl 2 After extraction, the organic layer was separated, concentrated and reprecipitated with petroleum ether to obtain a yellow solid powder with a yield of 67%. MS(EI)M + = 329, 1 H NMR (CDCl 3 , 300MHz): δ9.92(s, 3H), 7.84(d, 6H), 7.24(d, 6H).
[0033] (2) Add 0.4g (1.3mmol) of 4-bromo-1-bromomethylnaphthalene, 0.384g (1.4mmol) of triphenylphosphine and 10ml of xyl...
Embodiment 2
[0037] Example 2: Synthesis of 4,4'-bis(4-triphenylaminovinylnaphthyl-1-vinyl)triphenylamine.
[0038](1) Preparation of 1-bromo-4-(1-ethylenetriphenylamine)naphthalene: 4-bromo-1-bromomethylnaphthalene quaternary phosphonium salt (0.3g, 0.53mmol) obtained in step 2 of Example 1 and 4- N,N-Diphenylaminobenzaldehyde (0.08g, 0.3mmol) and sodium hydride (0.042g, 1.75mmol) were added to a 100ml three-necked flask, refluxed at 110°C for 6h, cooled, filtered, and the filtrate was taken, concentrated, petroleum Ether was the eluent and separated by column chromatography to obtain yellow 1-bromo-4-(1-ethylenetriphenylamine)naphthalene (0.064 g) with a yield of 40%. MS(EI)M + =476, elemental analysis (%): Cald. C, 75.63; H, 4.65; N, 2.94. Found.75.04; H, 4.16; N.3.28.
[0039] (2) 4,4'-bis(4-triphenylaminovinylnaphthyl-1-vinyl)triphenylamine preparation: under anhydrous and oxygen-free conditions, 0.253g (0.53mmol) 1-bromo-4- (1-ethylenetriphenylamine) naphthalene, 0.08g (0.27mmol) ...
Embodiment 3
[0042] Example 3: Synthesis of 4,4'-bis(4-triphenylaminovinylnaphthyl-1-vinyl)-N,N-diphenylaminobenzoic acid.
[0043] The synthetic method is similar to Example 2, only need to change N, N-bis(4-styrene) aniline in step 2 into N, N-bis(4-styrene)-4-aminobenzoic acid according to the obtained compound . Yield 21%, MS(MALDI)M + =1132.30. Elemental analysis (%): Cald.C, 88.03; H, 5.43; N, 3.71.Found.88.50; H.5.02; N.3.24.
[0044] The molecular formula of the obtained compound is,
[0045]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com