Method for detecting hydrogen peroxide or glucose based on enzyme simulation by ferroferric oxide magnetic nanometer particle
A technology of ferroferric oxide and nanoparticles, which is applied in the field of analytical chemistry, can solve the problems of time-consuming and labor-intensive preparation and purification, and the variability of natural enzymes, and achieve good selectivity, sensitivity, and good sensitivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The preparation steps of ferric oxide nanoparticle mimic enzyme are as follows:
[0038] (1) Mix 50mL concentration of 1M ferric chloride aqueous solution with 10mL concentration of 2M ferrous chloride hydrochloric acid solution (2M), and nitrogen gas for deoxygenation for 10 minutes;
[0039] (2) While vigorously stirring, the above-mentioned mixed solution was added dropwise to 500 mL of ammonia solution with a concentration of 0.7 M. The ammonia solution was deoxygenated and protected by nitrogen, and reacted at room temperature for 30 minutes, and the obtained black ferric oxide precipitated;
[0040] (3) Centrifuge the black iron ferric oxide precipitate obtained from the reaction, wash it with water three times; redisperse it in water to obtain ferric oxide nanoparticle-mimicking enzyme, and store it at room temperature for future use.
Embodiment 2
[0042] The steps for colorimetric detection of hydrogen peroxide by using iron ferric oxide nanoparticles to simulate enzymes are as follows:
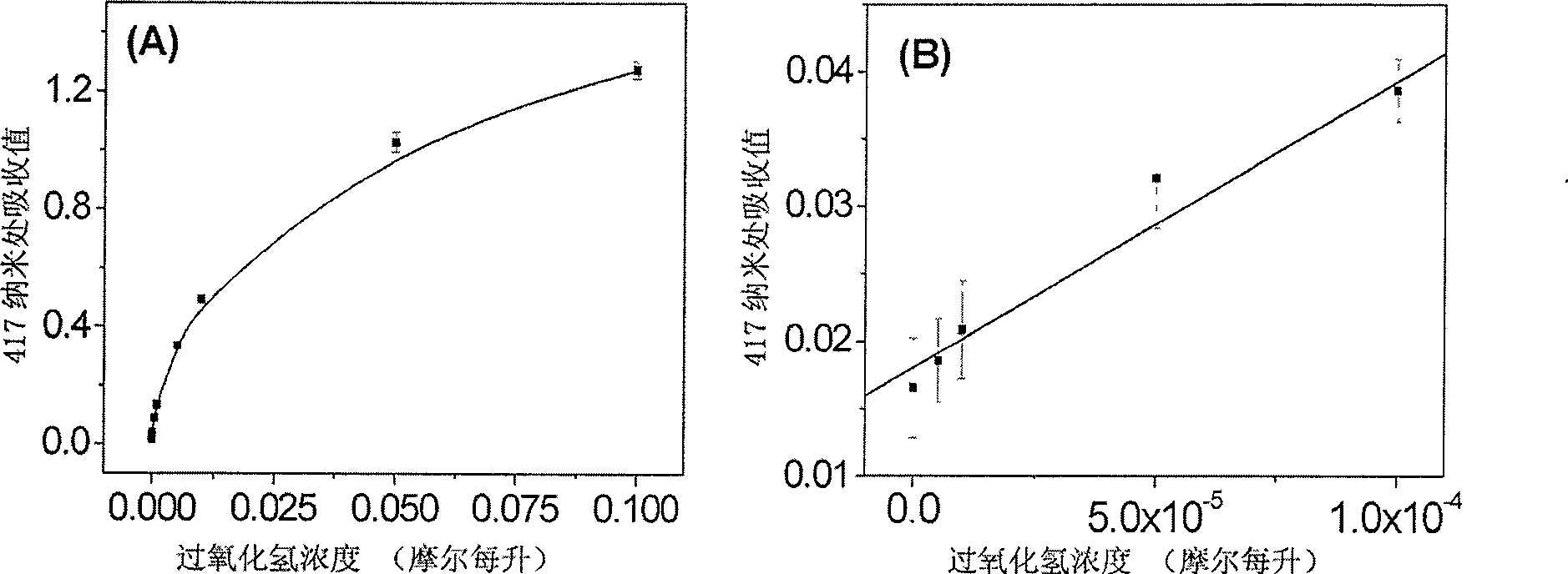
[0043] (1) 24 μL of 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt with a concentration of 60 mM, 10 μL ferric oxide nanoparticle mimic enzyme stock solution and 24 μL of 100 mM HO 2 o 2 Add to 185 μL of acetic acid buffer solution with a concentration of 0.2M and pH=4.0;
[0044] (2) React the mixed solution obtained in (1) in a water bath at 45°C for 10 minutes;
[0045] (3) removing the iron ferric oxide nanoparticle simulative enzyme in the reaction solution with an external magnetic field to obtain a reaction solution for removing the ferric oxide nanoparticle simulative enzyme;
[0046] (4) Add 100 μL of the reaction solution obtained in (3) to 900 μL of water, mix well, and measure the absorption spectrum.
Embodiment 3
[0048] The steps for colorimetric detection of glucose by using iron ferric oxide nanoparticles to simulate enzymes and glucose oxidase are as follows:
[0049] (1) Mix 20 μL of 20 mg / mL glucose oxidase with 200 μL of glucose solutions of different concentrations, and react at 37° C. for 30 minutes; the concentration of the glucose solution is 5×10 -5 , 1×10 -4 , 5×10 -4 , 1×10 -3 , 5×10 -3 and 1×10 -2 mol / L;
[0050] (2) Mix 24 μL of 60 mM 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt, 10 μL ferroferric oxide nanoparticle mimic enzyme stock solution and 800 μL concentration A 0.2M pH=4.0 acetate buffer solution was added to the 220 μL glucose reaction solution in the above step (1);
[0051] (3) The mixed solution in the above step (2) was reacted in a water bath at 45°C for 10 minutes, and the ferric oxide nanoparticles in the reaction solution were removed with an external magnetic field;
[0052] (4) Take 900 μL of the final reaction solutio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com