Ozagrel liposomes and preparation method thereof
A liposome and lipid technology, which is applied in the field of medicine, can solve the problems of short residence time in the body, shorten the administration time, and poor patient compliance, and achieve the effects of prolonging the residence time in the body, targeting, and controlling drug release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
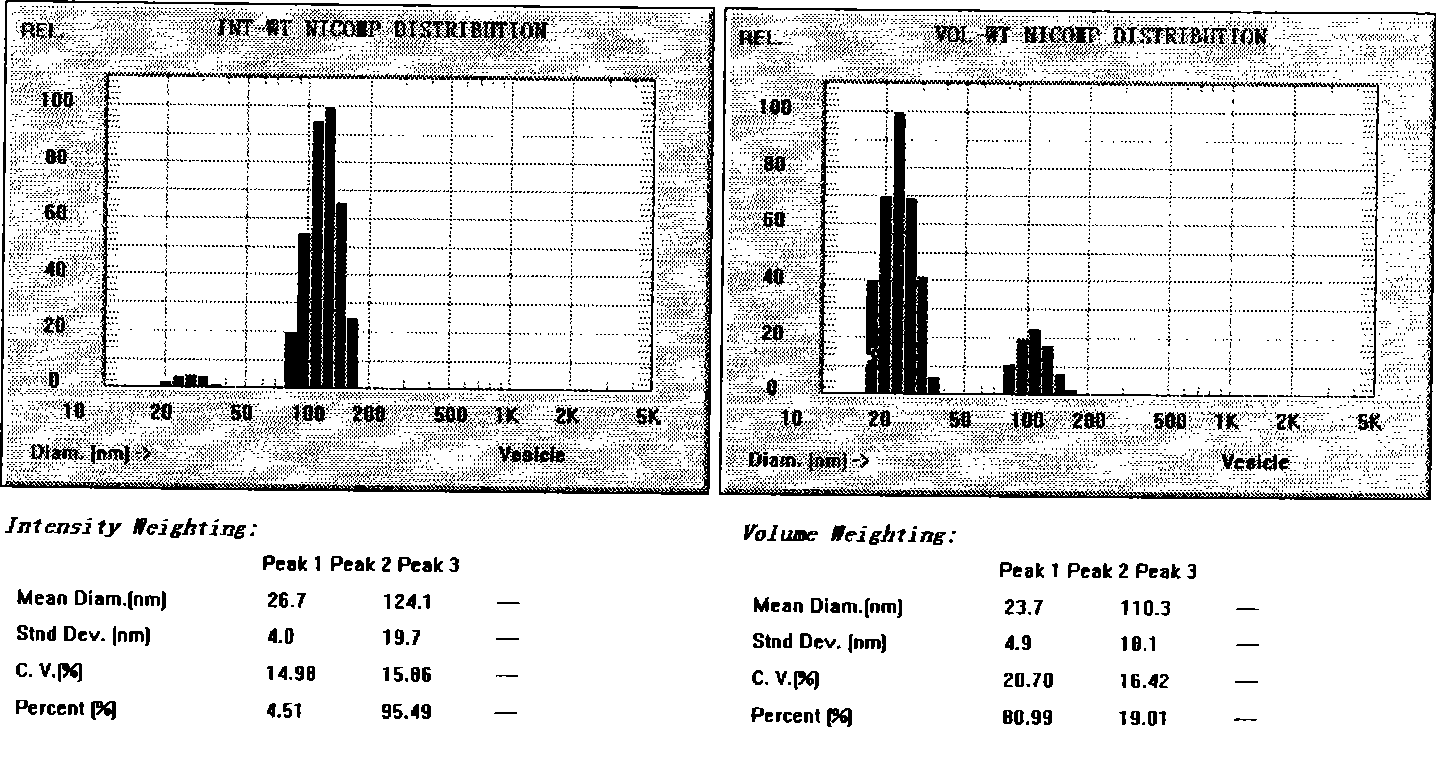
[0029] Take 5g of ozagrel, 30g of soybean lecithin, 30g of cholesterol, 200mL of chloroform, and add pH7.4 phosphate buffer to 1000mL. Put the above ozagrel, soybean lecithin, and cholesterol into a round-bottomed flask and fully dissolve them with chloroform, and place them in a constant temperature water bath at 20-40°C for rotary steaming under reduced pressure, so that the lipids form a uniform film at the bottom of the flask. Pour pH 7.4 phosphate buffer into the above-mentioned flask, hydrate, shake, then adjust the volume to 1000mL with pH 7.4 phosphate buffer, and homogenize 6 times through 750bar high-pressure emulsification to obtain a liposome suspension; or Add 200 g of glucose to the ozagrel liposome suspension, and freeze-dry to obtain ozagrel liposome solid powder.
Embodiment 2
[0031] Take 10g of ozagrel, 50g of egg yolk lecithin, 50g of cholesterol, and add pH7.4 phosphate buffer to 1000mL.
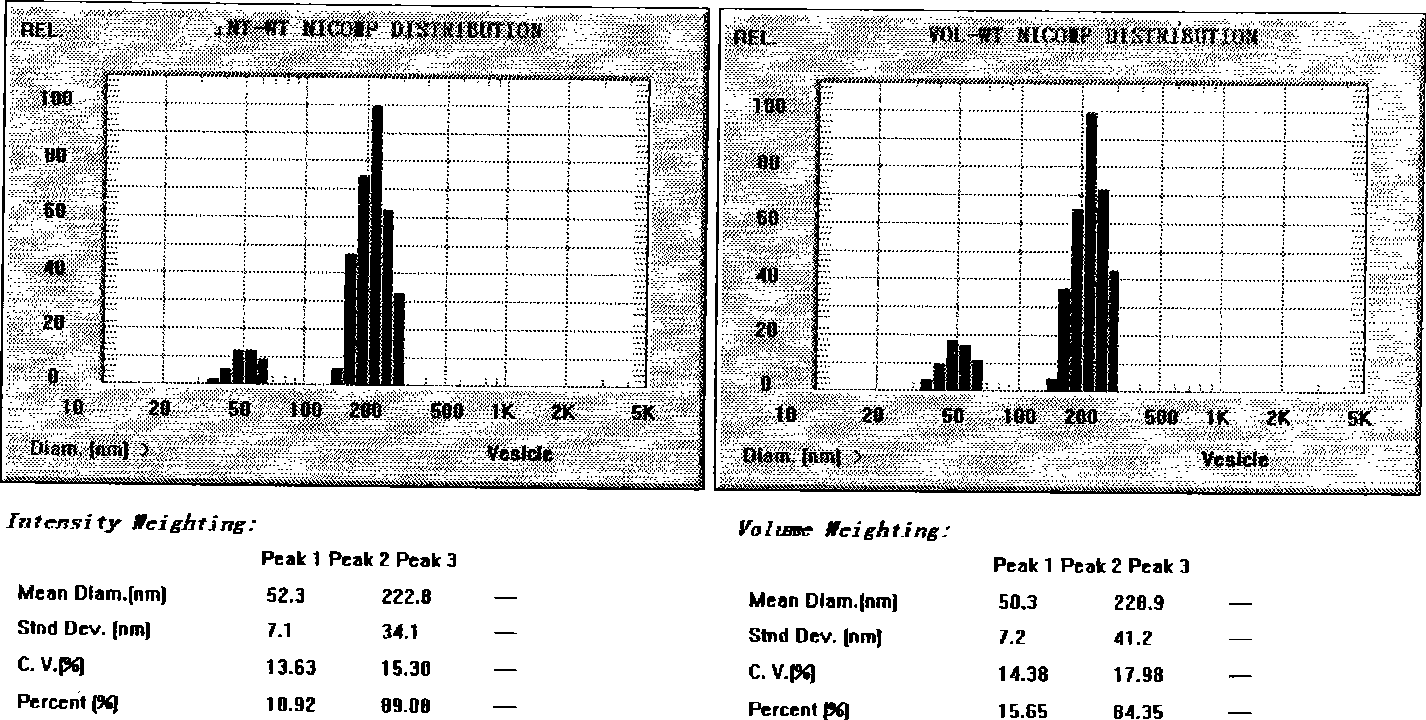
[0032] Dissolve the above-mentioned ozagrel, egg yolk lecithin, and cholesterol in an appropriate amount of ethanol, slowly inject into 800mL of pH7.4 phosphate buffer, remove the residual ethanol under reduced pressure, use the buffer to make up to 1000mL, and homogenize through 700bar high-pressure milk for 7 or 200 g of lactose was added to the above-mentioned ozagrel liposome suspension, and lyophilized to obtain ozagrel liposome solid powder.
Embodiment 3
[0034] Take 7.5g of ozagrel, 60g of dipalmitoylphosphatidylcholine, 50g of poloxamer F68, 50g of cholesterol, and add pH7.4 phosphate buffer to 1000mL.
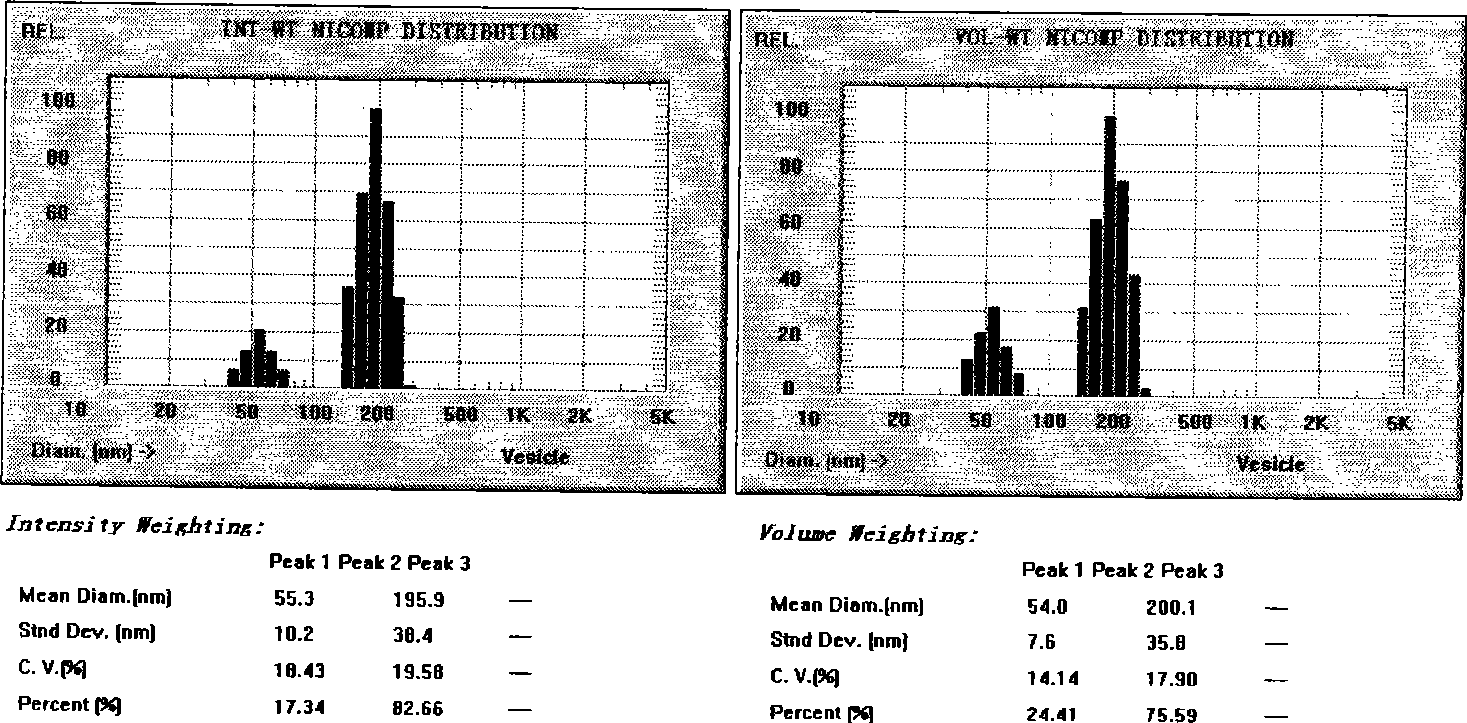
[0035] Take ozagrel, dipalmitoylphosphatidylcholine, poloxamer F68, and cholesterol, melt and mix until clear, drop into the pH7.4 phosphate buffer solution heated to 65°C under stirring, keep warm, after 500w, accumulate Ultrasonic dispersion for 5 minutes to prepare ozagrel liposome suspension; or add 100 g of trehalose to the above ozagrel liposome suspension, and spray dry to obtain ozagrel liposome solid powder. Spray drying conditions: inlet temperature 160°C, outlet temperature 91°C, air volume 100%, flow rate 0.01%, yield 40.8%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com