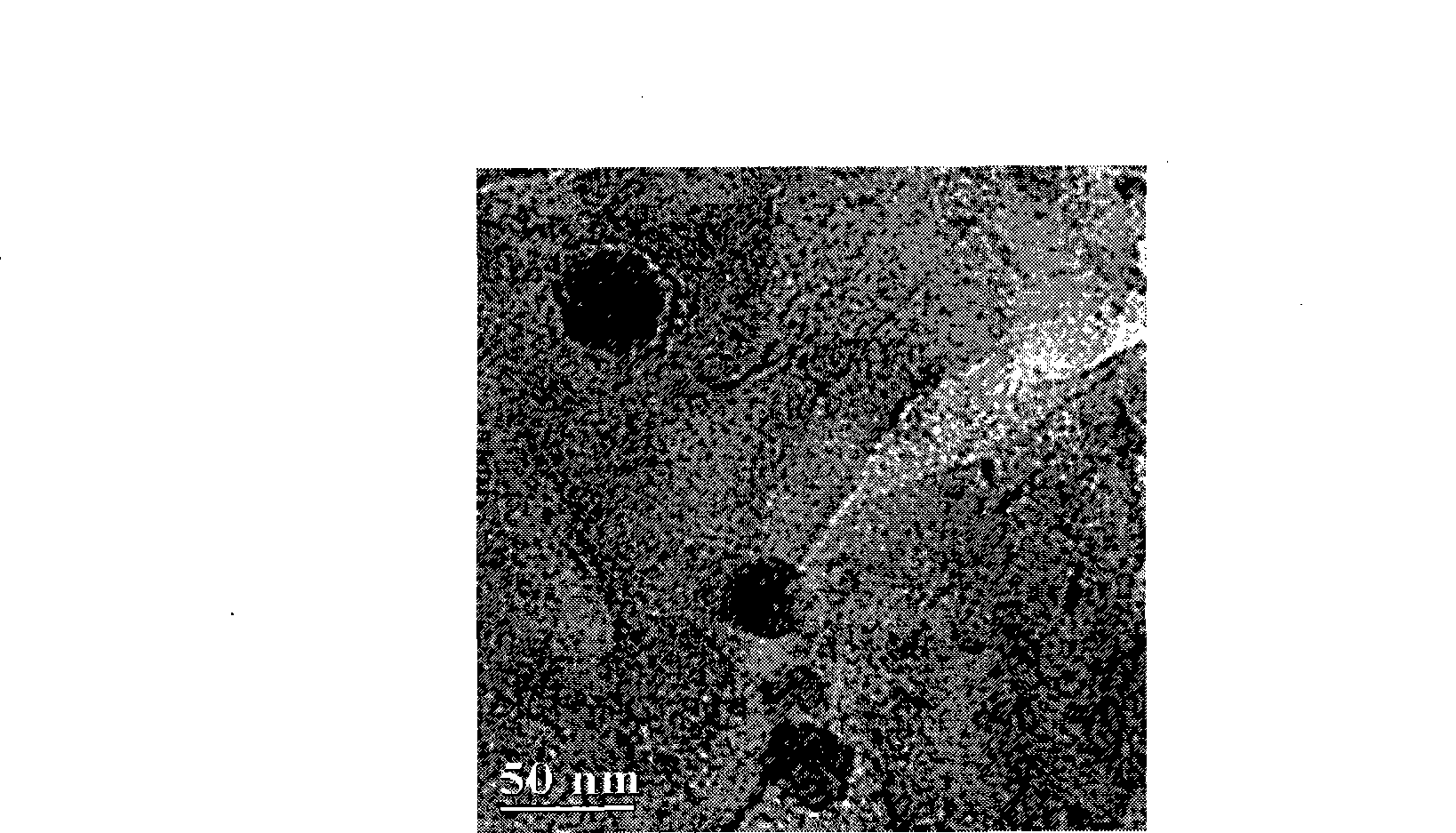
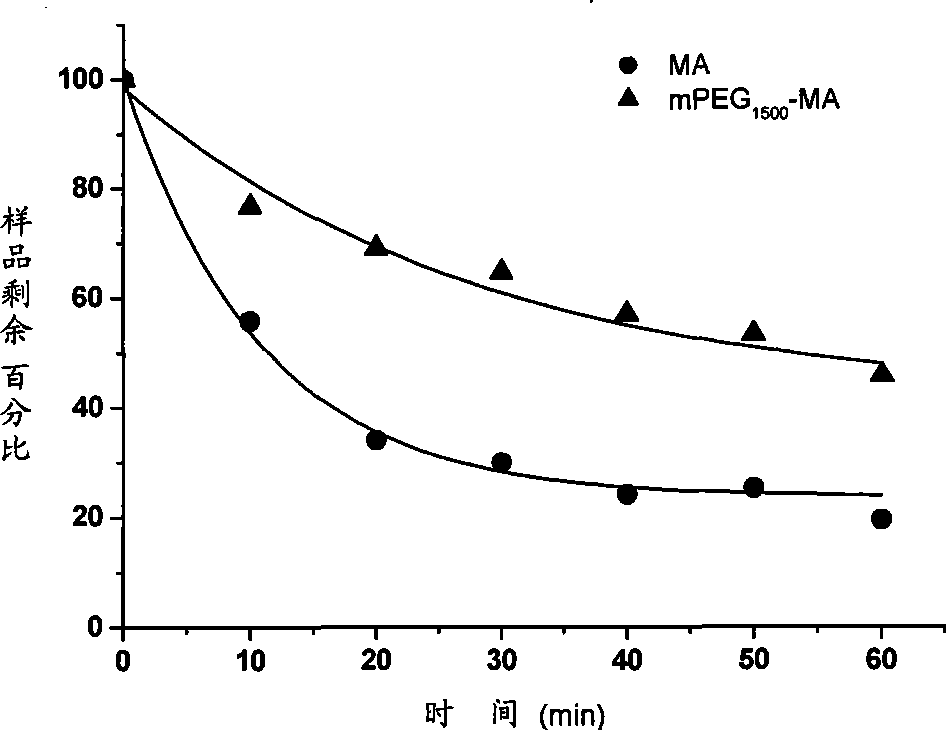
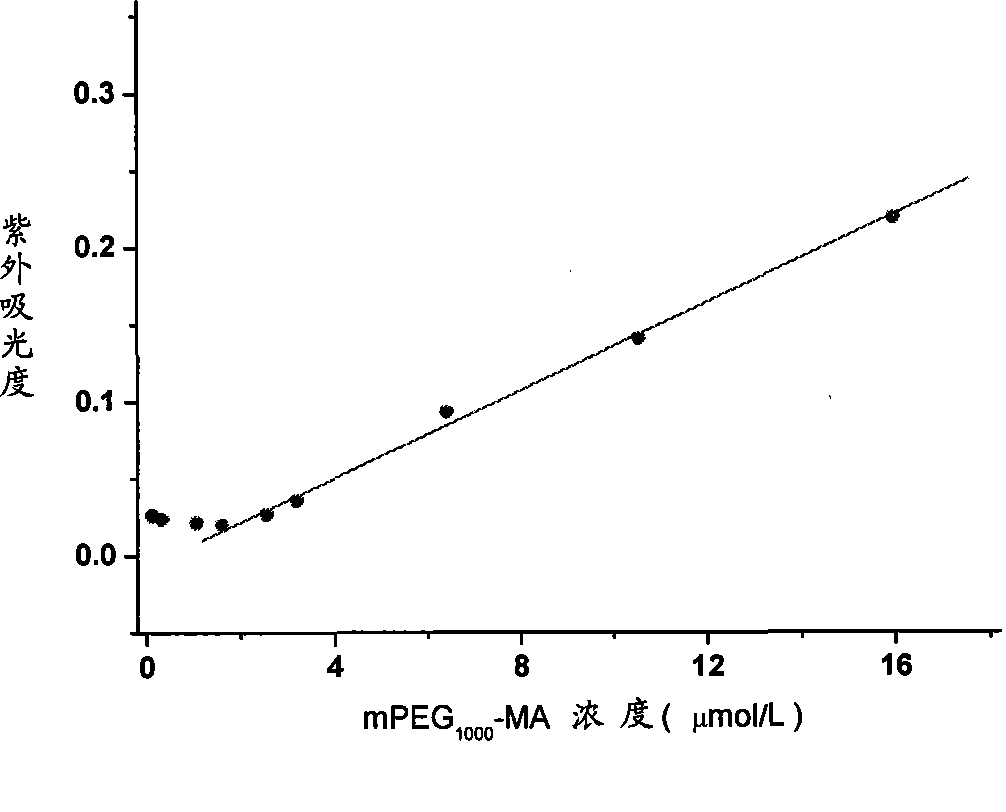Polyglycol modified antimicrobial peptide and uses thereof
A polyethylene glycol and antibacterial peptide technology, applied in the field of modification of peptides, can solve the problems such as the reduction of antibacterial activity of antibacterial peptides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Synthesis of polypeptide MA resin with side chain protecting groups: add 1g Rinkamide MBHA resin (0.74mmol / g) to a 50mL reaction bottle, add 15mL dimethylformamide (DMF) to swell for 10 minutes, remove the solvent by suction filtration, then add 15mL 20% piperidine / DMF solution, stirred for 30 minutes, and suction filtered. The resin was washed 6 times with DMF, and the solvent was removed by suction filtration. Add 1.44g Fmoc-Arg(Pbf)-OH (Pbf is 2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl), 0.46g (2.22mmol) DIC to the reaction flask (N,N-Diisopropylcarbodiimide) and 0.30g (2.22mmol) HOBt (1-hydroxybenzotriazole) and 15mL DMF were stirred at room temperature to react. After 2 hours of reaction, a small amount of resin was taken for ninhydrin color test, and the result showed that the condensation reaction was complete. Suction filtration, wash the resin 6 times with DMF, and remove the solvent by suction filtration. The first (protected) amino acid at the C-ter...
Embodiment 2
[0015] The preparation of polypeptide MA: the polypeptide MA resin 0.5g that embodiment 1 obtains is joined in the 50mL round-bottomed flask, adds 10mLK reagent (trifluoroacetic acid / water / phenol / sulfide anisole / 1,2-dimercaptoethylene glycol =82.5 / 5 / 5 / 5 / 2.5, volume ratio), stirred at room temperature for 4 hours. Filter and collect the filtrate. The resin was washed three times with trifluoroacetic acid. The filtrates were combined, and most of the trifluoroacetic acid was removed by rotary evaporating the filtrate, and 8-10 times the volume of ether was added to precipitate the polypeptide MA, and the ether precipitate was placed in the refrigerator overnight. Centrifuge to remove ether, and dry in vacuo to obtain crude polypeptide MA.
[0016]Dissolve 200mg of polypeptide MA crude product in 5mL of pure water, and purify by preparative reverse-phase HPLC. , volume ratio), the flow rate is 12mL / min. The collected solution was concentrated by rotary evaporation, and then f...
Embodiment 3
[0018] Polyethylene glycol carboxyl derivatives (mPEG 500 -COOH) Preparation: Mix 3.2g of mPEG-OH with an average molecular weight of 500, 20g of distilled water and 0.4g of KOH, cool to 0.5°C with an ice-salt bath, and slowly add 0.4g of acrylonitrile dropwise. The solution was stirred at 0-5°C for 2 hours. Slowly add 30g of concentrated sulfuric acid dropwise, and heat at 95-100°C for 3 hours. After cooling to room temperature, add 200 mL of distilled water and extract with dichloromethane 3 times, 30 mL each time. Dichloromethane extract with anhydrous Na 2 SO 4 After drying, the solvent was evaporated under reduced pressure to obtain mPEG 500 -COOH.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com