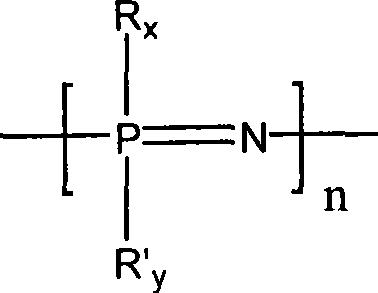
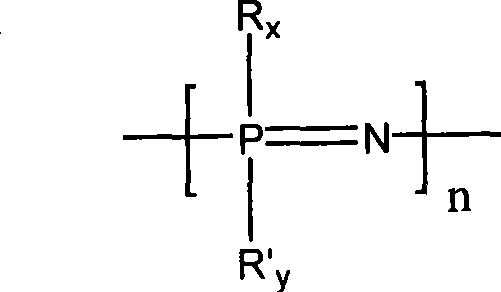
Glucose responding type polyphosphazene hydrogel and preparation method thereof
A glucose-responsive, polyphosphazene technology, used in pharmaceutical formulations, medical preparations with inactive ingredients, metabolic diseases, etc., can solve the problem of poor material degradation performance, failure to achieve the best effect of drug treatment, and difficulty in satisfying drug release, etc. problem, to achieve the effect of good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
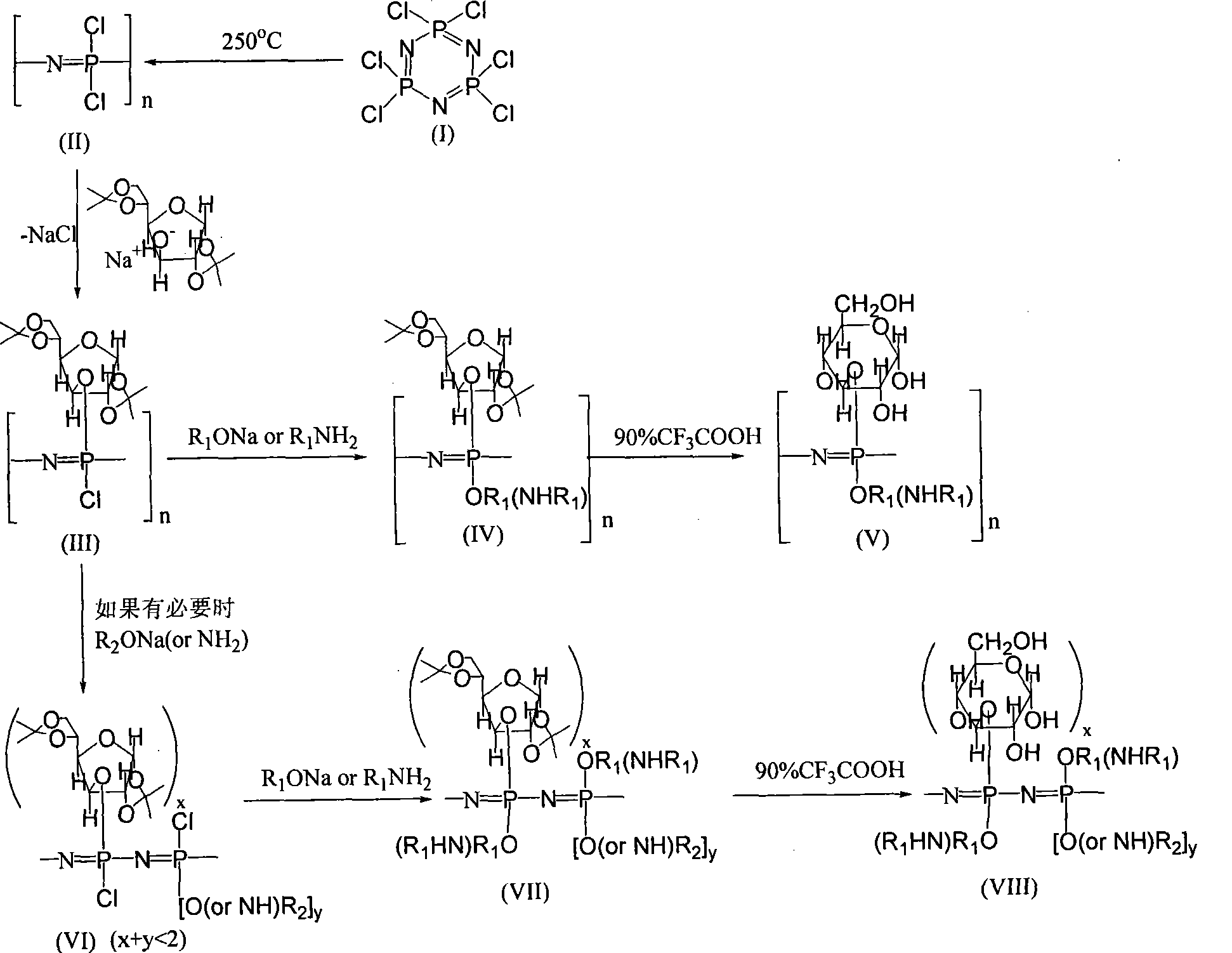
[0026] (1) Preparation of polydichlorophosphazene: 2 g of hexachlorocyclotriphosphazene was sealed in a vacuum glass tube, and reacted at 260° C. for 48 hours. After cooling to room temperature, wash repeatedly with hot dry petroleum ether to remove unreacted monomers and macrocyclic phosphazene by-products, then fully dissolve the residue with dry benzene, filter to remove insoluble matter, and precipitate with petroleum ether , and vacuum-dried to obtain polydichlorophosphazene for use.
[0027] (2) Preparation of 1,2:5,6-di-O-isopropylidene-α-D-glucose: 5g of anhydrous α-D-glucose and 57ml of anhydrous acetone were placed in a Dry the there-necked flask, successively add 3.79g of anhydrous zinc chloride and 0.23ml of 85% phosphoric acid, react at room temperature for 30 hours, filter, and add sodium hydroxide (25mol / L) solution dropwise to the filtrate until neutral. After filtering again, the filtrate was distilled off under reduced pressure to obtain a viscous liquid. T...
Embodiment 2
[0034] Step (1), (2) are the same as embodiment 1
[0035] (3) Preparation of polyphosphazene:
[0036] Place 100ml of dry tetrahydrofuran solution in a three-necked flask with a mechanical stirrer, then add 7.80g (0.03mol) of 1,2:5,6-di-O-isopropylidene-α-D-glucose and 0.75 g (0.033mol) metal sodium, reacted at room temperature for 24 hours. 1.16 g (0.01 mol) of polydichlorophosphazene solution was slowly added dropwise to the above solution, and refluxed for 96 hours. Then a tetrahydrofuran solution (100ml) of sodium methylethoxide (prepared by 0.80ml (0.01mol) methoxyethanol and 0.25g (0.011mol) metallic sodium in tetrahydrofuran at room temperature for 24 hours) was slowly added dropwise to the above solution, After reacting at room temperature for 24 hours, the temperature was raised to 50° C. and the reaction was continued for 24 hours. After centrifuging to remove insoluble matter, remove most of the solvent by rotary evaporation to obtain a viscous liquid, precipita...
Embodiment 3
[0040] Step (1), (2) are the same as embodiment 1
[0041] (3) Preparation of polyphosphazene:
[0042] Place 100ml of dry tetrahydrofuran solution in a three-necked flask with a mechanical stirrer, then add 5.20g (0.02mol) of 1,2:5,6-di-O-isopropylidene-α-D-glucose and 0.50 g (0.022mol) metal sodium, reacted at room temperature for 24 hours. 1.16 g (0.01 mol) of polydichlorophosphazene solution was slowly added dropwise to the above solution, and refluxed for 96 hours. Then a tetrahydrofuran solution (100ml) of sodium methylethoxide (prepared by 1.60ml (0.02mol) methoxyethanol and 0.50g (0.022mol) metallic sodium in tetrahydrofuran at room temperature for 24 hours) was slowly added dropwise to the above solution, After reacting at room temperature for 24 hours, the temperature was raised to 50° C. and the reaction was continued for 24 hours. After centrifuging to remove insoluble matter, remove most of the solvent by rotary evaporation to obtain a viscous liquid, precipita...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com