Method for synthesizing diphosphine monoxide ligand
A technology of single oxides and synthesis methods, applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc., can solve the difficulties of product separation, low yield and selectivity, and have not been solved etc. to overcome the low selectivity and yield and simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
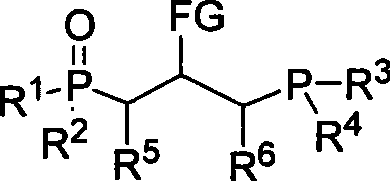
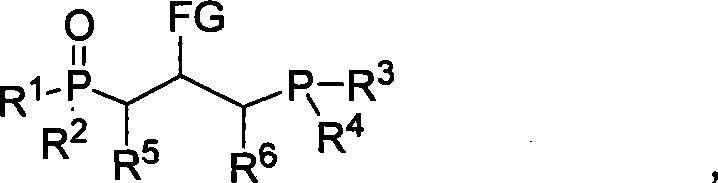
[0025] The synthesis of embodiment 1. bisphosphine monoxide, R in the general structural formula 1 =R 2 =R 3 =R 4 = Ph, R 5 =R 6 =H, FG=CO 2 Et.
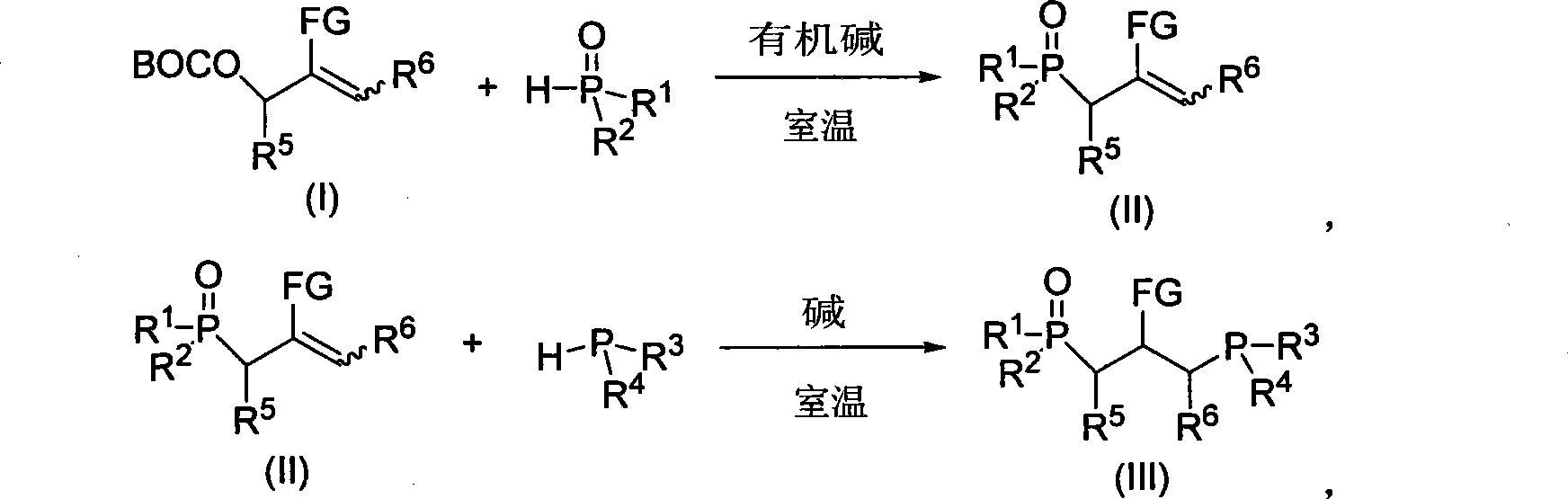
[0026] 1) In a 25mL round bottom bottle with a magnetic stirring bar, add 5mL of tetrahydrofuran solvent, tert-butyl allyl carbonate (R 5 =R 6 =H, FG=CO 2 Et, prepared from formaldehyde and ethyl acrylate) 108 mg (ie 0.5 mmol), diphenylphosphine oxide 121 mg (ie 0.6 mmol) and organic base catalyst 1,4-diazabicyclo [2.2.2] octane 11 mg (i.e. 0.1 mmol); the resulting reaction mixture was stirred at room temperature for 24 hours, then added 5 mL of water and stirred for 0.5 hours, then extracted the reaction mixture twice with 20 mL of dichloromethane, combined the dichloromethane extracts, dried over anhydrous magnesium sulfate, After filtration, the solvent is removed to obtain the primary product of monophosphine oxide synthon; the primary product is purified by silica gel column chromatography, that is, silica gel is used ...
Embodiment 2
[0028] The synthesis of embodiment 2. bisphosphine monoxide, R in the general structural formula 1 =R 2 =R 3 =R 4 = Ph, R 5 = 4-NO 2 C 6 h 4 , R 6 =H, FG=CO 2 Et.
[0029] Synthetic steps and process parameters are basically the same as in Example 1, and the differences are listed as follows:
[0030] 1) The raw material tert-butyl allyl carbonate used is R 5 = 4-NO 2 C 6 h 4 , R 6 =H, FG=CO 2 Et, (prepared from p-nitrobenzaldehyde and ethyl acrylate), the dosage was 168mg (ie 0.5mmol), and 198mg of light yellow liquid monophosphine oxide synthon was obtained, with a yield of 91%;
[0031] 2) Reaction of 218 mg (ie 0.5 mmol) of the monophosphine oxide synthon obtained in the previous step with 93 mg (ie 0.5 mmol) of diphenylphosphine and 34 mg (ie 0.5 mmol) of sodium ethoxide to obtain a colorless oily product bisphosphine Single oxide 242 mg, yield 76%. The structure and purity of this product are confirmed by nuclear magnetic resonance spectrum, and its struct...
Embodiment 3
[0032] Embodiment 3. the synthesis of bisphosphine monoxide, R in the general structural formula 1 =R 2 =R 3 =R 4 = Ph, R 5 = 2-furyl, R 6 =H, FG=CO 2 Et.
[0033] 1) In a 25mL round bottom bottle with a magnetic stirring bar, add 5mL of toluene solvent, tert-butyl allyl carbonate (R 5 = 2-furyl, R 6 =H, FG=CO 2 Et, prepared from furfural and ethyl acrylate) 141mg (ie 0.5mmol), diphenylphosphine oxide 121mg (ie 0.6mmol) and organic base catalyst 1,4-diazabicyclo[2.2.2]octane 11mg (i.e. 0.1 mmol), the resulting reaction mixture was stirred at room temperature for 24 hours, then 5 mL of water was added and stirred for 0.5 hour, then the reaction mixture was extracted twice with 20 mL of dichloromethane, the combined dichloromethane extracts were dried over anhydrous magnesium sulfate, After filtration, the solvent is removed to obtain the primary product of monophosphine oxide synthon; the primary product is purified by silica gel column chromatography, that is, silica ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com