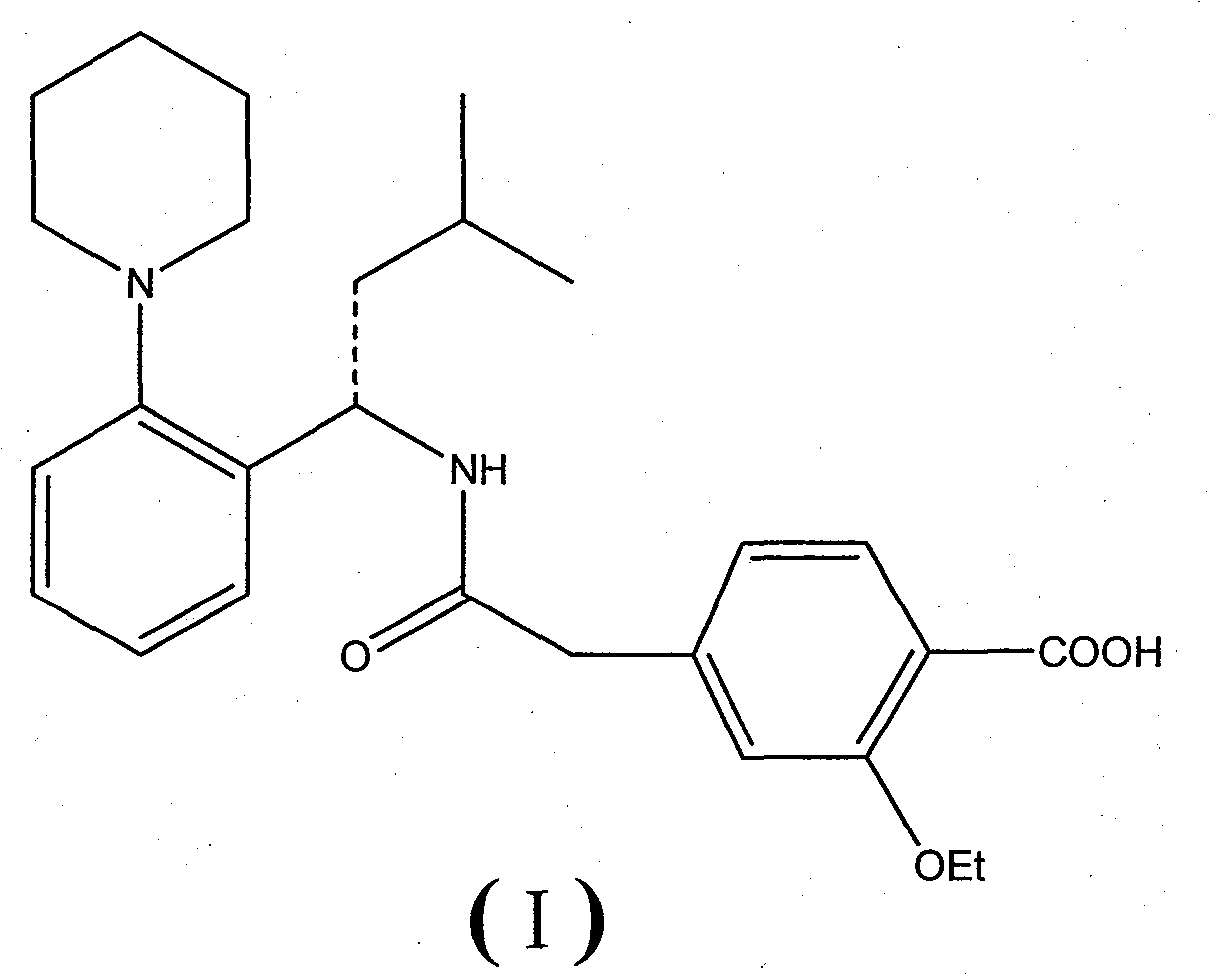
Method for preparing repaglinide
A compound and the selected technology are applied in the field of preparation of the drug repaglinide, which can solve problems such as unfavorable industrial production, high toxicity, and long reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Preparation of (S)-N-(4-methoxybenzyl)-3-methyl-1-[2-(1-piperidinyl)phenyl]butyl-1-amine
[0034] The raw material 3-methyl-1-(2-piperidin-1-yl)phenyl)-1-butanone (3.3Kg, 13.4mol), toluene (30L), p-toluenesulfonic acid (330g), 4- Methoxybenzylamine (4.5Kg, 32.5mol) was put into the reaction kettle, and refluxed to separate water for five hours. About (1.6Kg) of anhydrous sodium sulfate was added, and the reaction was continued overnight. The next day, the reaction solution was cooled to room temperature, poured into saturated sodium bicarbonate solution (20L), separated into layers, the aqueous layer was extracted with ethyl acetate (10L), the organic layers were combined, dried, and concentrated to dryness to obtain an oil: 8.0Kg .
[0035] TLC: petroleum ether / ethyl acetate=20 / 1, Rf starting material=0.7, Rf product=0.3
[0036] Put the previous step oil (8.0Kg), methanol (30L), nickel chloride hexahydrate (7Kg) into the reaction kettle, and cool to 0°C. Sodium bo...
Embodiment 2
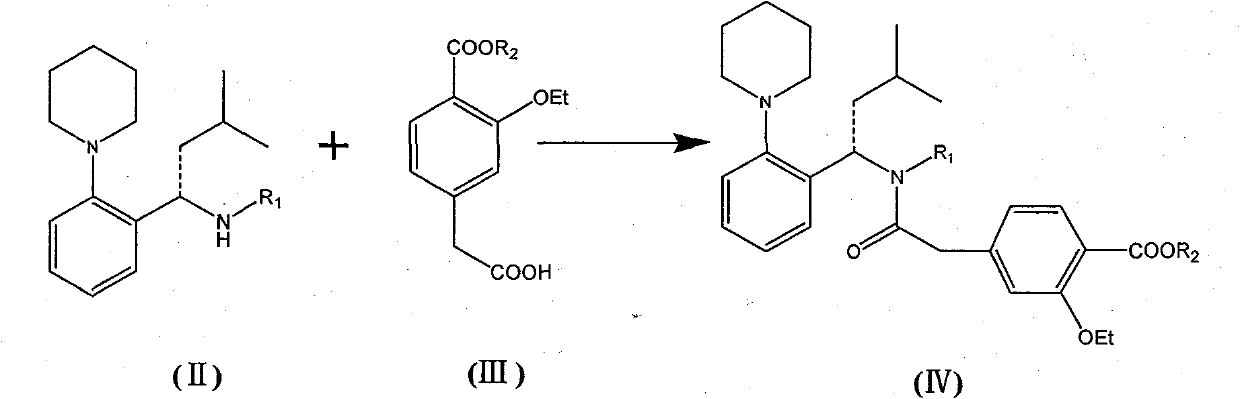
[0043] (S)-4-(2-((4-methoxybenzyl)(3-methyl-1-(2-(1-piperidinyl)phenyl)butyl)amino)-2-oxo Preparation of ethyl)-2-ethoxy ethyl benzoate
[0044] Dry (S)-N-(4-methoxybenzyl)-3-methyl-1-[2-(1-piperidinyl)phenyl]butyl-1-amine toluene in the previous step , CDI (640g), side chain acid 2-(3-ethoxy-4-(ethoxycarbonyl) phenyl) acetic acid (750g) were dropped in the 50L reaction kettle, added acetonitrile (4L), room temperature stirring reaction 8 Hour. Add water (6L) and stir, separate the layers, extract the aqueous layer with ethyl acetate (6L×2), combine the organic layers, wash with saturated brine, and dry over anhydrous magnesium sulfate. After filtration, the filtrate was concentrated to dryness under reduced pressure, and the residue was purified by column chromatography (eluent: dichloromethane / methanol=100:1), and qualified components were concentrated to dryness to obtain an oil: 1.34Kg.
[0045] TLC: petroleum ether: ethyl acetate = 3: 1, Rf raw material = 0.6, Rf produ...
Embodiment 3
[0048] (S)-2-ethoxy-4-{2-[(3-methyl-1-(2-(1-piperidinyl)phenyl)butyl)amino]-2-oxoethyl} Preparation of Benzoic Acid (Repaglinide)
[0049] 1) Alkaline hydrolysis
[0050] Put intermediate 3 (1.34Kg) of the previous step, methanol (8L), and aqueous sodium hydroxide solution (sodium hydroxide / water=560g / 2.2L) into a 20L reactor, and heat to reflux for 2 hours. Concentrate under reduced pressure to remove methanol, adjust the pH of the residue to 4-5 with 10% hydrochloric acid, extract with dichloromethane (2.8 L×2), combine the organic layers, and dry over anhydrous magnesium sulfate. After filtration, the filtrate was concentrated to dryness under reduced pressure, and the obtained intermediate was directly used for feeding in the next step.
[0051] TLC: petroleum ether / ethyl acetate=3 / 1, Rf product=0.3, Rf raw material=0.8;
[0052] 2) Acid hydrolysis
[0053]Trifluoroacetic acid (5.4 L) was added to the intermediate of the upward step, and after stirring for 20 minutes, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com