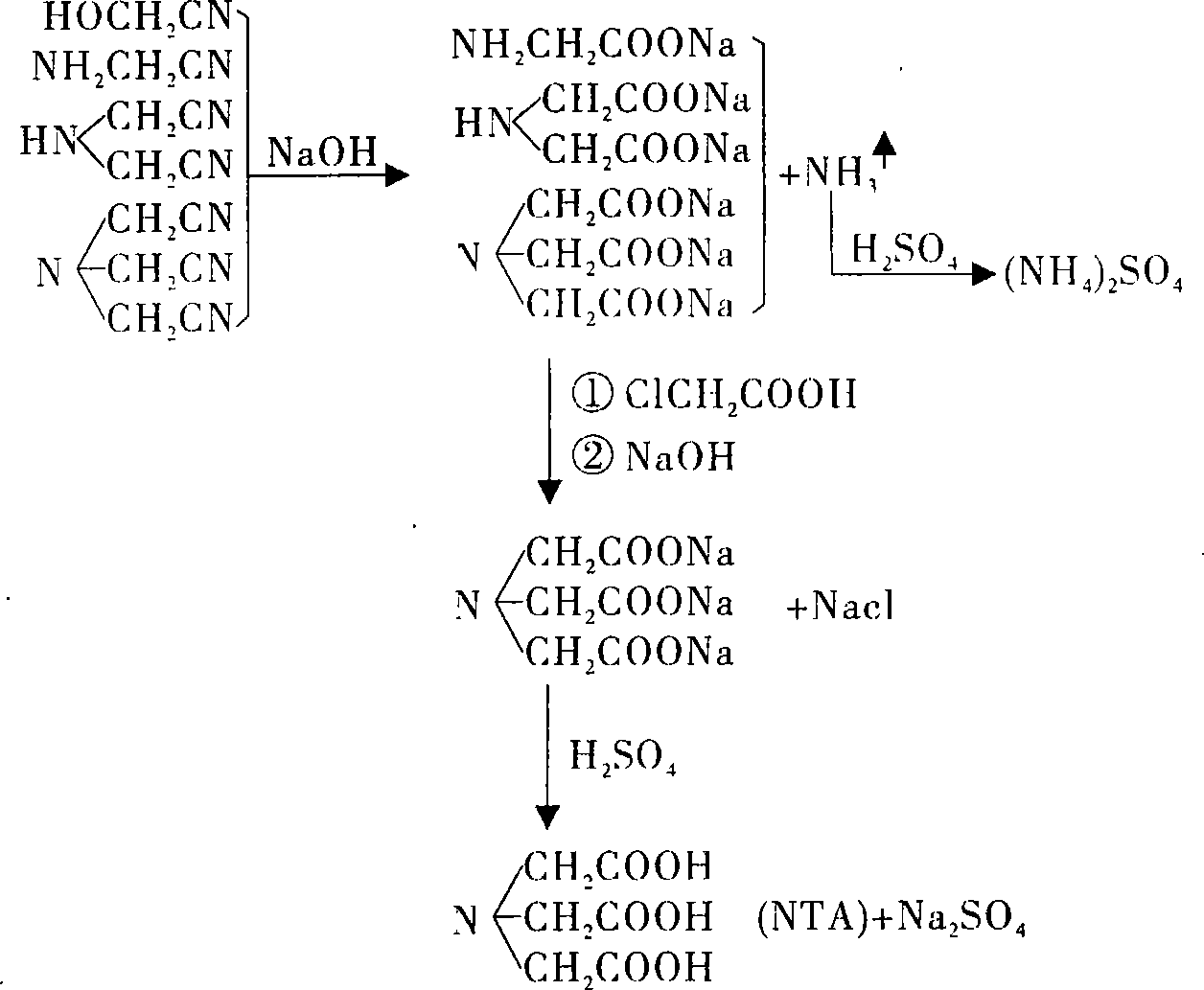Method for synthesizing nitrilotriacetic acid by raffinate obtained from iminodiacetonitrile by hydrocyanic acid method
A technology of iminodiacetonitrile and nitrilotriacetic acid, applied in chemical instruments and methods, cyanide reaction preparation, organic compound preparation, etc., can solve the problems of high processing cost, rare HCN raw materials, high production cost, etc., and achieve safe operation The effects of low requirements, thorough treatment methods, and few types and quantities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Measure the raffinate of 500ml IDAN (containing 50.4g / l of hydroxyacetonitrile, 162.1g / l of aminoacetonitrile, 37.3g / l of iminodiacetonitrile, 19.3g / l of nitrilotriacetonitrile, which is thicker in black) and add 863g of 30% NaOH solution, Stir evenly, gradually heat to 115°C for hydrolysis, the ammonia produced by hydrolysis is absorbed with 40% sulfuric acid solution to generate ammonium sulfate. Hydrolyze for 4.5 hours, cool and add appropriate amount of water to dilute to obtain 800ml of hydrolyzate [containing glycine (GLY) 177.1g / l, iminodiacetic acid (IDA) 32.7g / l, nitrilotriacetic acid (NTA) 17.2g / l], in Add 375.6g (100%) chloroacetic acid at 15°C, adjust the pH of the reaction solution to about 11 with 30% liquid alkali, then raise the temperature to 55-60°C, and keep the reaction for 5 hours. During the heat preservation reaction process, use 30% liquid caustic soda to adjust the pH of the reaction solution to 11 to 12. After the reaction is completed, cool an...
Embodiment 2
[0027] Measure 500ml of the hydrolyzed solution after hydrolysis of IDAN residue with KOH (containing GLY210.1g / lIDA50.4g / l NTA26.3g / l pH=9.6), add KOH131.6g and cool to 20°C, add chloroacetic acid 282.5g, stir Heat up to 50-60°C, keep stirring for 1 hour, keep using KOH to keep the pH of the reaction solution greater than 9 during the heat-retaining reaction process, use 195g of KOH, after the reaction is completed, cool at 20-30°C and acidify with 31% hydrochloric acid, the pH of the acidified solution is 1.0 , cooled and crystallized for 2 hours, suction filtered and washed at 23° C. to obtain 465 g of NTA wet product with a content of 62.8%. Based on GLYIDANTA in the hydrolyzate, the yield was 92.1% of the theoretical yield. The filtrate is neutralized with KOH, decolorized, concentrated, crystallized, and dried to obtain potassium chloride solid, which is used as a raw material for potash fertilizer.
[0028] Suspend the above NTA wet product in 1000ml of water, heat to 6...
Embodiment 3
[0030] Inject 282LIDAN raffinate in 1500L enamel reaction kettle with the hydrolyzed solution after NaOH hydrolysis, solution pH=11.3 (containing GLY 119.3g / l IDA 107.6g / l NTA 56.6g / l), then add 30.2% liquid caustic soda 298KG, Slowly add 110kg of chloroacetic acid (96.5%) at 20-25°C under stirring and cooling, after the addition is complete, stir for 20 minutes, then slowly heat to 50-60°C, keep warm for 4 hours, take samples and analyze and calculate, the conversion rate of chloroacetic acid is 101.2% . Cool the reaction solution, add concentrated sulfuric acid for acidification, when the pH=4 or so, NTA solids start to precipitate, after acidification to pH=0.8, stop adding acid, cool and crystallize for 4.5 hours, and centrifuge to get 200.2KG gray-black solid, NTA content 69.3% %, based on the GLY IDA NTA in the hydrolyzate, the wet product yield is 95.5%, the centrifuged mother liquor is black, add acidic activated carbon 12KG, decolorize at room temperature for 12 hours...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com