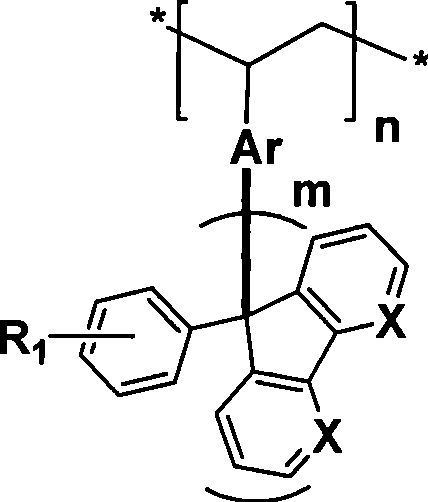
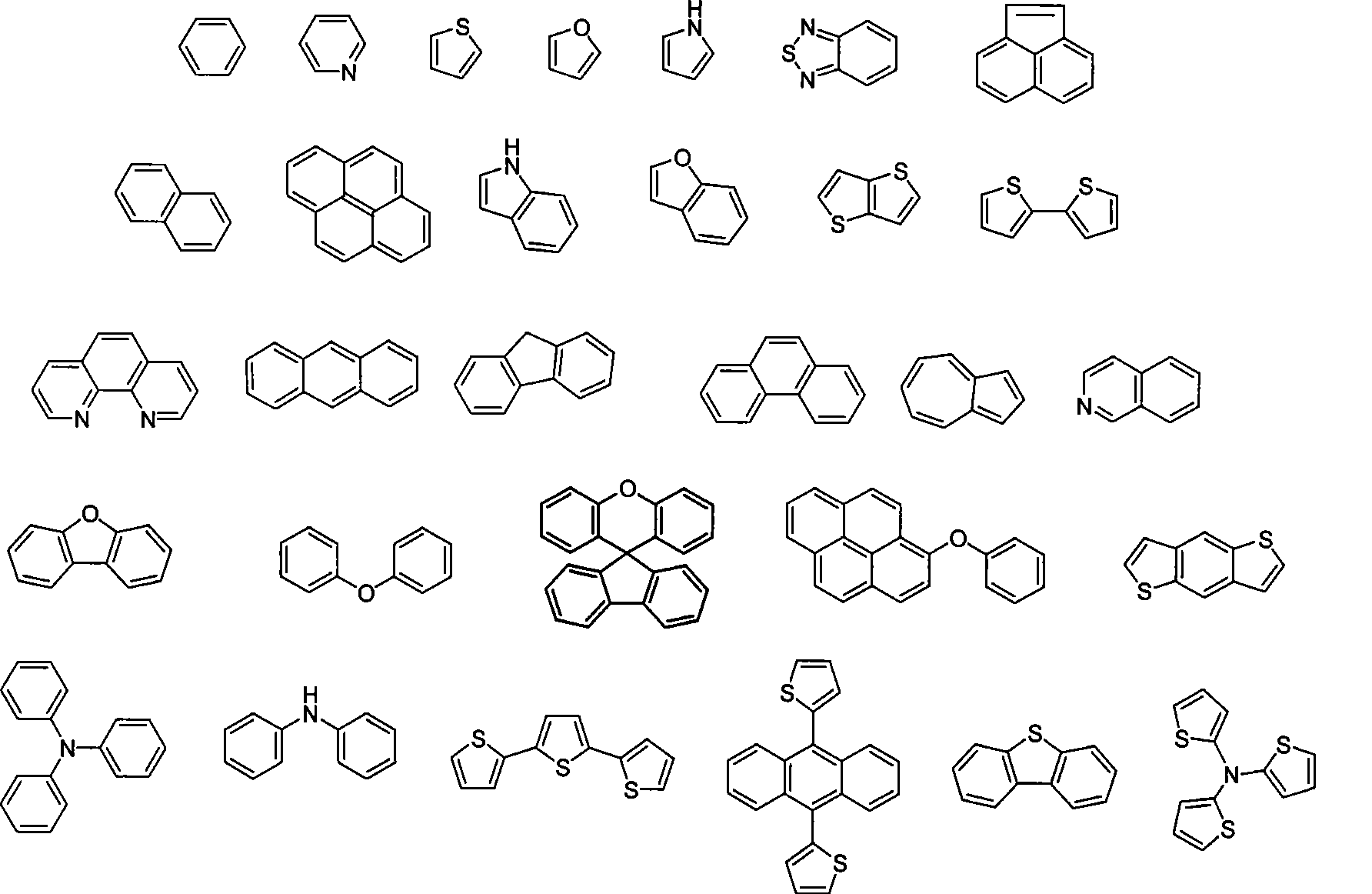
Poly (diaryl fluorene ethylene) material, as well as preparation and use method thereof
A technology of vinyl diaryl fluorene and ethylene, which is applied in the field of organic optoelectronic materials, can solve the problems that high-performance polymer optoelectronic materials have not been seen, and achieve the effects of mild monomer synthesis conditions, easy-to-obtain raw materials, and simple preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0062] In the preparation method of poly(diaryl fluorene vinyl) material, at first by preparing vinyl diaryl fluorene monomer, then carry out free radical polymer preparation, following three examples represent three kinds of different methods of preparing monomer, specific as follows:
[0063] (1) Poly(phenylthienylfluorene vinyl) material
[0064]
[0065] Wherein, step 1. reacts under room temperature, boron trifluoride diethyl ether complex catalyst / dichloromethane; Bromosuccinimide reaction; step ③ preparation of Grignard reagent under anhydrous, anaerobic, nitrogen protection, magnesium / tetrahydrofuran conditions, and then dropwise adding dry N,N-dimethylformamide; step ④ using Wei Tixi Reaction Trityl phosphine bromide and potassium carbonate are dissolved in 1,4-dioxane to change the aldehyde group into a vinyl group; step ⑤ at a temperature of 85°C-95°C, benzoyl peroxide is used as a catalyst / Polyvinyl alcohol is used as a stabilizer, and the phenylthienyl fluo...
Embodiment 1
[0074] Embodiment 1, poly(diphenylfluorene ethylene) material preparation:
[0075] 9-Phenyl-fluoren-9-ol
[0076] 9-phenyl-9H-fluoren-9-ol
[0077] Experimental procedure: Take 0.66ml, 6.3mmol of bromobenzene and 1.506g, 6.3mmol of magnesium to react to generate Grignard reagent, and dissolve it in 50mL of tetrahydrofuran, and react with 1.134g, 6.3mmol of fluorenone at 60°C for 24 hours to form a large amount of white precipitate. Finally add saturated NHCl 4 Convert Grignard salts to alcohols. After the reaction was completed, extracted with ether, dried and rotary evaporated, and purified on a silica gel column with a mixed solvent of petroleum ether:dichloromethane (3:2) to obtain a slightly pale yellow solid tertiary alcohol (90% yield). GC-MS (EI-m / z): 258 (M + ). 1 H NMR (400MHz, CDCl 3 , ppm): δ 7.691-7.672 (d, J=7.6Hz, 2H), 7.406-7.325 (m, 6H), 7.292-7.236 (m, 5H), 2.508 (s, 1H). 13 C NMR (CDCl 3 , ppm): δ 150.658, 143.391, 139.82, 129.339, 128.698, 128.459, ...
Embodiment 2
[0094] Embodiment 2, poly (diphenylamine phenyl fluorene vinyl) material preparation:
[0095] N-phenyl-4-(9-phenyl-fluoren-9-yl)aniline
[0096] N-phenyl-4-(9-phenyl-9H-fluoren-9-yl)benzonamine
[0097] Experimental procedure: Dissolve 1.624g, 6.3mmol of 9-phenyl-fluorene-9-ol and 1.599g, 9.45mmol of diphenylamine in 60ml of dichloromethane according to the equivalent ratio of 1:1.5, and add dropwise 12.6ml of trifluoro Reaction of boron-diethyl ether complex for 30 minutes, adding 20ml of ethanol and 20ml of water to quench the reaction, extracting with dichloromethane (20ml×3), drying and rotary evaporation, purification with petroleum ether silica gel column, and recrystallization with tetrahydrofuran and petroleum ether to obtain the product (97% yield). GC-MS (EI-m / z): 409 (M + ).
[0098] N-(2-chloroethyl)-N-phenyl-4-(9-phenyl-fluoren-9-yl)aniline
[0099] N-(2-chloroethyl)-N-phenyl-4-(9-phenyl-9H-fluoren-9-yl)benzonamine
[0100] Experimental procedure: Mix 6.9ml...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Film thickness | aaaaa | aaaaa |
| Resistance | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com