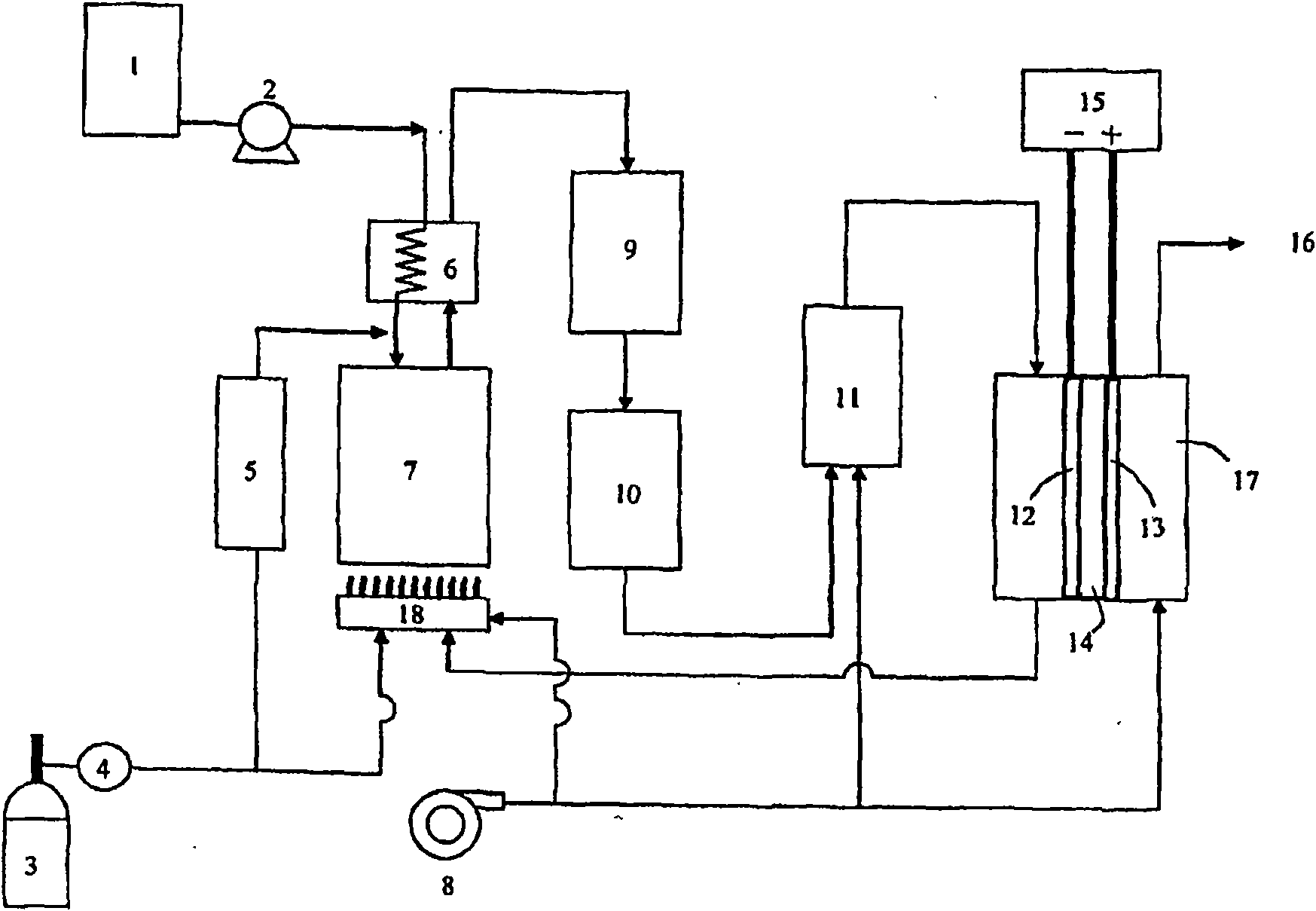
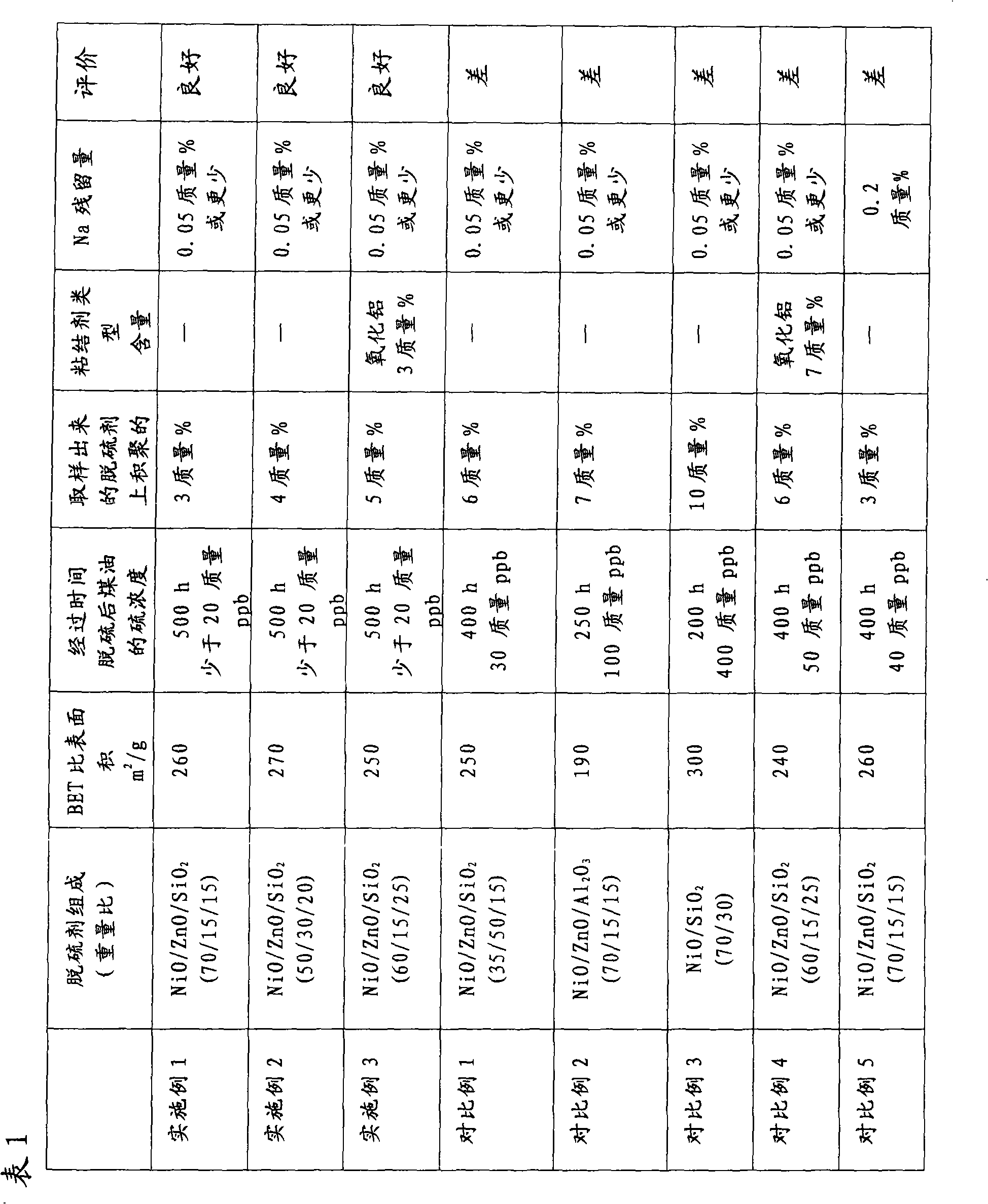
Desulfurizing agent for kerosene, desulfurization method and fuel cell system using the desulfurizing agent for kerosene
A technology of fuel cell system and desulfurizer, which is applied in the direction of fuel cells, fuel cell additives, chemical instruments and methods, etc., can solve the problems of desulfurization function decline, achieve the effects of inhibiting degradation and improving durability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] 272.5 g of nickel nitrate hexahydrate (commercially available special grade reagent) and 54.8 g of zinc nitrate hexahydrate (commercially available special grade reagent) were dissolved in ion-exchanged water to prepare 2500 ml of an aqueous solution, hereinafter referred to as liquid A. 130.8 g of sodium carbonate (commercially available special-grade reagent) was dissolved in ion-exchanged water, and it was mixed with 50 g of commercially available silica sol (particle diameter: about 15 nm, silica content: 15.0 g) to 1000 ml of solution was prepared, hereinafter referred to as Liquid B. Liquid A and Liquid B were mixed at a temperature of 40°C while stirring to form a precipitate. After washing the precipitate with ion-exchanged water, the obtained cake was crushed, then dried at a temperature of 120° C. for 10 hours and calcined at a temperature of 360° C. for 4 hours, thereby yielding 100 g of a calcined powder. The calcined powder has the following composition: w...
Embodiment 2
[0076] 166.6 g of nickel acetate tetrahydrate (commercially available special grade reagent) and 80.9 g of zinc acetate dihydrate (commercially available special grade reagent) were dissolved in ion-exchanged water to prepare 3000 ml of an aqueous solution, hereinafter referred to as liquid A. 121.0 g of sodium carbonate (commercially available special-grade reagent) was dissolved in ion-exchanged water, and it was mixed with 66 g of commercially available silica sol (particle diameter: about 15 nm, silica content: 20.0 g) to 1200 ml of solution was prepared, hereinafter referred to as Liquid B. Liquid A and Liquid B were mixed at a temperature of 40°C while stirring to form a precipitate. After washing the precipitate with ion-exchanged water, the resulting cake was crushed, then dried at a temperature of 120° C. for 10 hours and calcined at a temperature of 360° C. for 4 hours, thereby yielding 100 g of a calcined powder. The calcined powder has the following composition, w...
Embodiment 3
[0079] 233.6 g of nickel nitrate hexahydrate (commercially available special grade reagent) and 54.8 g of zinc nitrate hexahydrate (commercially available special grade reagent) were dissolved in ion-exchanged water to prepare 2500 ml of an aqueous solution, hereinafter referred to as liquid A. 115.1 g of sodium carbonate (commercially available special-grade reagent) was dissolved in ion-exchanged water, and it was mixed with 83 g of commercially available silica sol (particle diameter: about 15 nm, silica content: 25.0 g) to 1200 ml of solution was prepared, hereinafter referred to as Liquid B. Liquid A and Liquid B were mixed at a temperature of 40°C while stirring to form a precipitate. After washing the precipitate with ion-exchanged water, the resulting cake was crushed, then dried at a temperature of 120° C. for 10 hours and calcined at a temperature of 360° C. for 4 hours, thereby yielding 100 g of a calcined powder. The calcined powder has the following composition, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com