Di-demethoxycurcumin precursor liposome and preparation method thereof
A technology of double demethoxy curcumin and double demethoxy curcumin, applied in the fields of biomedicine and targeted preparations, can solve the problems of leakage, fusion, and no disclosure of in vivo data of solid dispersion preparations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] The specific contents of the high-efficiency jet mixing method provided by the invention are as follows:
[0076] (1) The influence of the lipid solution and the flow rate of the hydration medium: Weigh the medicine and the lipid film material (phospholipid and phospholipid derivatives, cholesterol and its derivatives) according to the optimal prescription amount and dissolve them in ethanol, and the fixed lipid solution flow rate is 10mL / min, adjust the flow rate of the hydration medium, measure the encapsulation efficiency and particle size of the liposome, the results are shown in Table 7:
[0077] The influence of table 7 lipid liquid and hydration medium flow velocity ratio on liposome (n=6)
[0078]
[0079] The results show that the flow rate of the hydration medium is more than 10 times that of the lipid solution, and the particle size of the formed lipid vesicles is smaller. Due to the increase of the flow rate of the polar aqueous phase, the drug-containin...
Embodiment 2
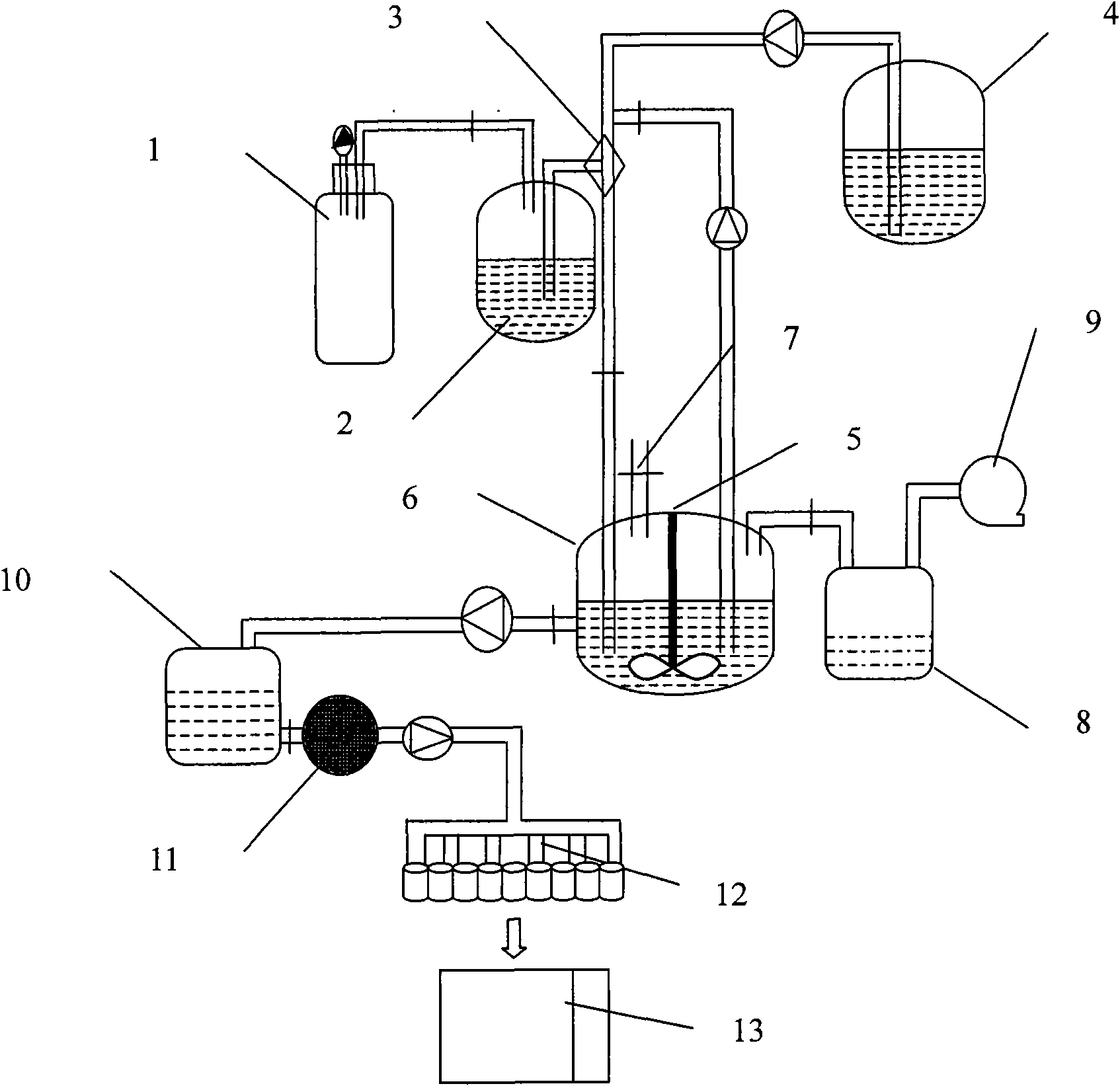
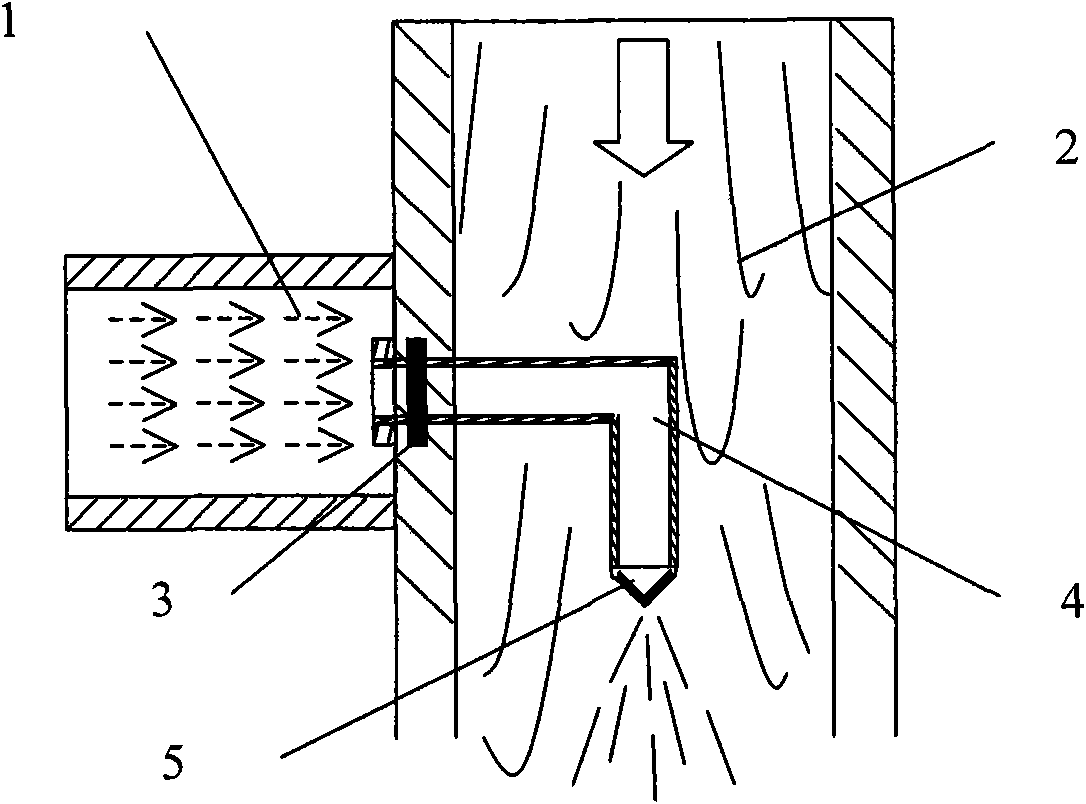
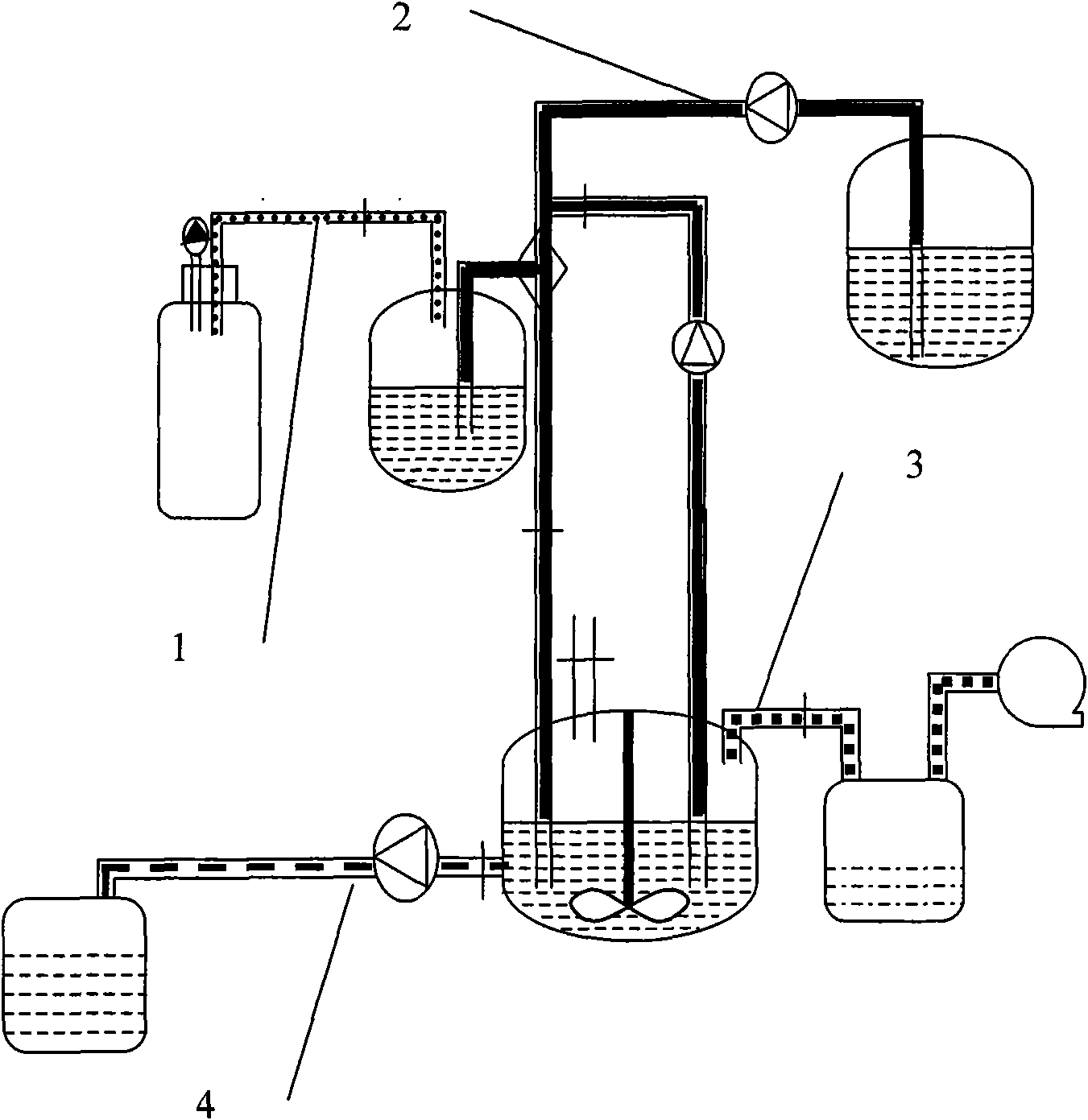
[0085] Weigh 16.0g of soybean lecithin, 6.0g of cholesterol and 0.75g of bis-demethoxycurcumin and ultrasonically dissolve them in 85mL of ethanol, put them into the lipid liquid tank 2, and dissolve 1.0g of poloxamer 188 in 980mL of pH6. 5. Phosphate buffer solution (hydration medium), which is packed into the hydration solution tank 4. Turn on the high-pressure irrigation 1 nitrogen, flow the drug-containing lipid liquid into the jet mixer 3, set the flow rate to 10mL / min, and form fine stream droplets through the small holes of the 250μm nozzle; at the same time, turn on the high-pressure pump of the hydration liquid irrigation 4, and the flow rate is 280mL / min, the hydration medium and the lipid alcohol solution are sprayed and mixed continuously and efficiently, and the mixed solution is poured into the tank 6, and the lipid vesicles are formed by automatic stirring and mixing. Open the circulation pipeline, continue high-speed circulation and mixing until the two phases...
Embodiment 3
[0088] Weigh 15.0g of hydrogenated soybean lecithin, 5.0g of cholesterol and 0.7g of bis-demethoxycurcumin and ultrasonically dissolve them in 80mL of ethanol, and put them into the lipid liquid tank 2; Put water into the hydration fluid tank 4. Turn on the high-pressure irrigation 1 nitrogen, flow the drug-containing lipid liquid into the jet mixer 3 at a flow rate of 20mL / min, and inject the liquid droplets into the circulation pipeline in a thin stream through the small hole of the 250μm nozzle, and turn on the high-pressure pump of the hydration fluid irrigation 4 at the same time , the flow rate is 280mL / min, the hydration medium and the lipid alcohol solution are sprayed and mixed continuously and efficiently, and the mixed solution is poured into the tank 6, and the lipid vesicles are formed by automatic stirring and mixing. Open the circulation pipeline, continue high-speed circulation and mixing until the two phases are completely dispersed, open the organic solvent r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com