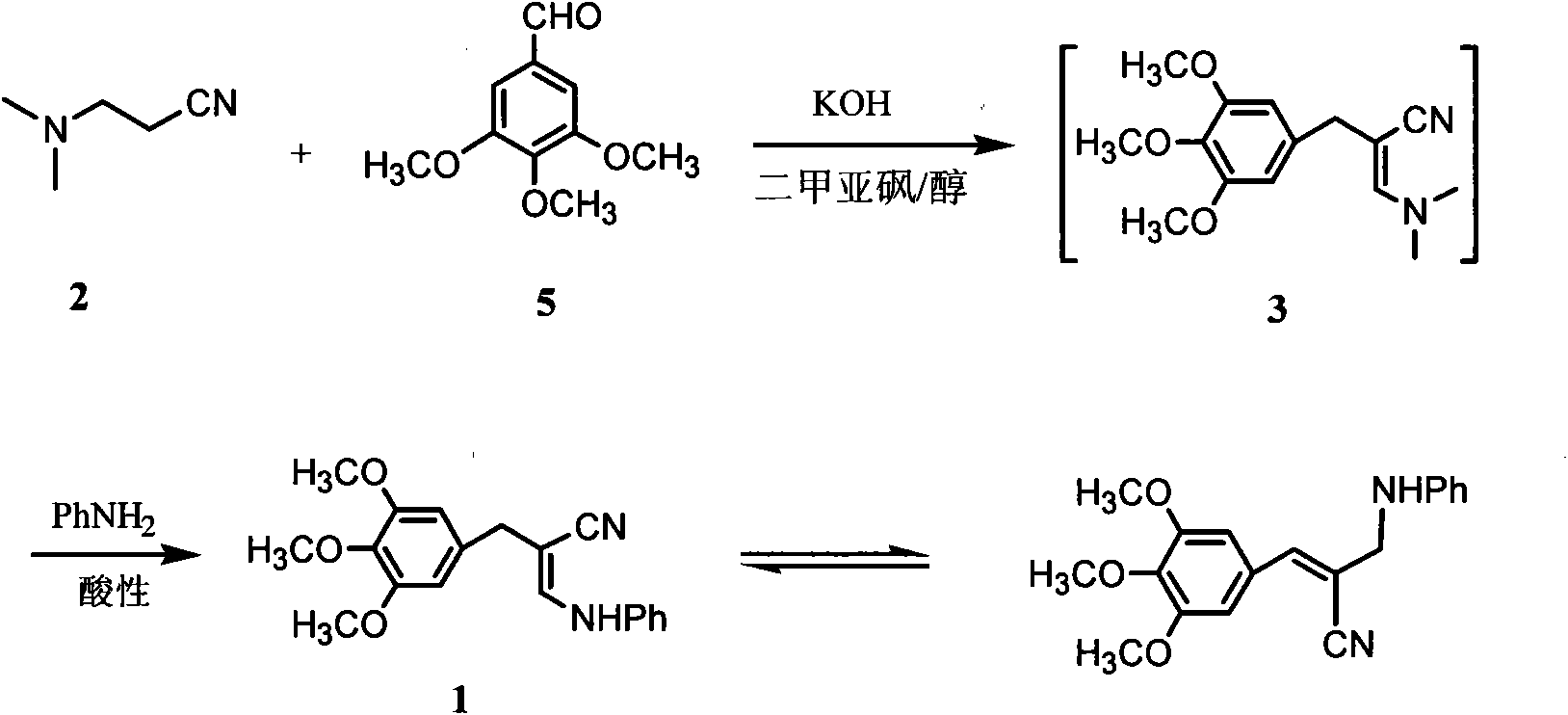
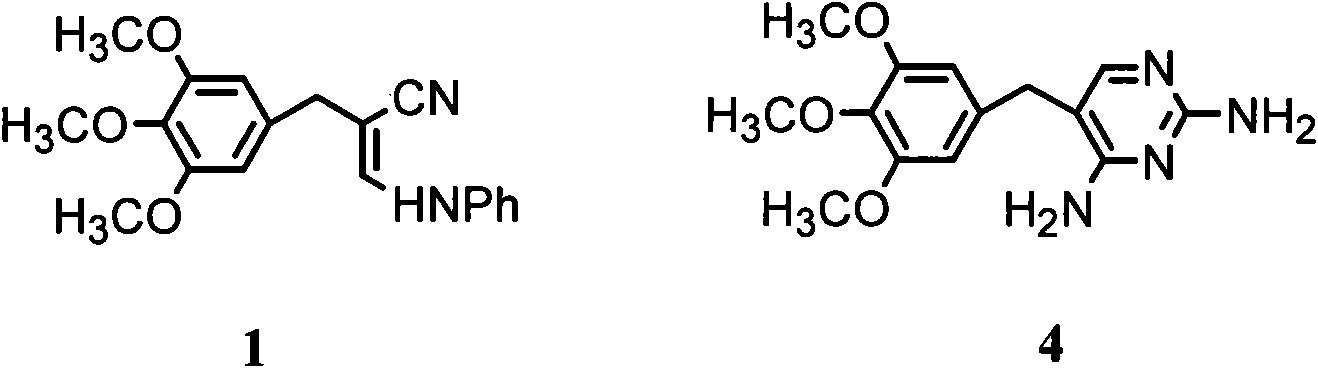Preparation method of 3- anilino-2-(3,4,5-trimethoxy benzyl) acrylonitrile
A technology of trimethoxybenzyl and dimethylaminopropionitrile, which is applied in the field of drug synthesis, can solve the problems of high production cost and low yield, and achieve low production cost, high yield and easy industrial scale production Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Add 250mL of dimethyl sulfoxide, 127.6g of 3-dimethylamine propionitrile (1.30mol) and 11.8g (0.21mol) of potassium hydroxide and 50mL of methanol into a three-necked flask equipped with mechanical stirring. The prepared solution was fully stirred at 45°C for 0.5 hours. At this temperature, 196.2 g of TMB (1.0 mol) was added in batches within 1 hour, then the temperature was raised to 85° C. and the reaction was continued with stirring for 4 hours, followed by thin layer chromatography (TLC) until the spots of TMB raw material disappeared. Cool to 40°C, add 250mL of methanol to stir and dilute under stirring, then add 98.7g of aniline (1.06mol), add 2mol / L dilute sulfuric acid dropwise within 1 hour until the pH is in the range of 3.0-3.5, heat up and reflux for reaction After 2 hours, TLC followed until the 3 spots disappeared. Methanol was distilled off under reduced pressure, 250 mL of water was added, and the reaction mixture was frozen to 3°C. A large amount of ye...
Embodiment 2
[0028] Add 250mL of dimethyl sulfoxide, 127.6g of 2 (1.30mol) and a solution of 11.8g (0.21mol) of potassium hydroxide and 50mL of tert-butanol in sequence in a three-necked flask equipped with mechanical stirring. Stir well for 0.5 hours. At this temperature, 196.2 g of TMB (1.0 mol) was added in batches within 1 hour, then the temperature was raised to 85° C. and the reaction was continued with stirring for 4 hours, followed by TLC until the spots of TMB raw material disappeared. Cool to 40°C, add 250mL of tert-butanol under stirring and dilute, then add 98.7g of aniline (1.06mol), add 2mol / L dilute sulfuric acid dropwise within 1 hour until the pH is in the range of 3.0-3.5, and heat up Reflux reaction for 2 hours, followed by TLC until the spot of 3 disappeared. The tert-butanol was distilled off under reduced pressure, 250 mL of water was added, and the reaction mixture was frozen to 3°C. A large amount of yellow precipitate precipitated and was filtered. The filter cak...
Embodiment 3
[0030] In a three-necked flask equipped with mechanical stirring, 250 mL of dimethyl sulfoxide, 50 mL of methanol, 127.6 g of 2 (1.30 mol), 11.8 g (0.21 mol) of potassium hydroxide and 196.2 g of TMB (1.0 mol) were added successively, The reaction was fully stirred at 80° C. for 5 hours, followed by TLC until the spots of TMB raw material disappeared. Cool to 40°C, add 250mL of methanol to stir and dilute under stirring, then add 98.7g of aniline (1.06mol), add 2mol / L dilute sulfuric acid dropwise within 0.5 hours until the pH is in the range of 3.0-3.5, heat up and reflux for reaction After 2 hours, TLC followed until the 3 spots disappeared. Methanol was distilled off under reduced pressure, 250 mL of water was added, and the reaction mixture was frozen to 3°C. A large amount of yellow precipitate precipitated and was filtered. The filter cake was washed with 500 mL of 15% (v / v) cold ethanol aqueous solution, and 289.3 g of light yellow solid 1 was obtained after vacuum dryin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com