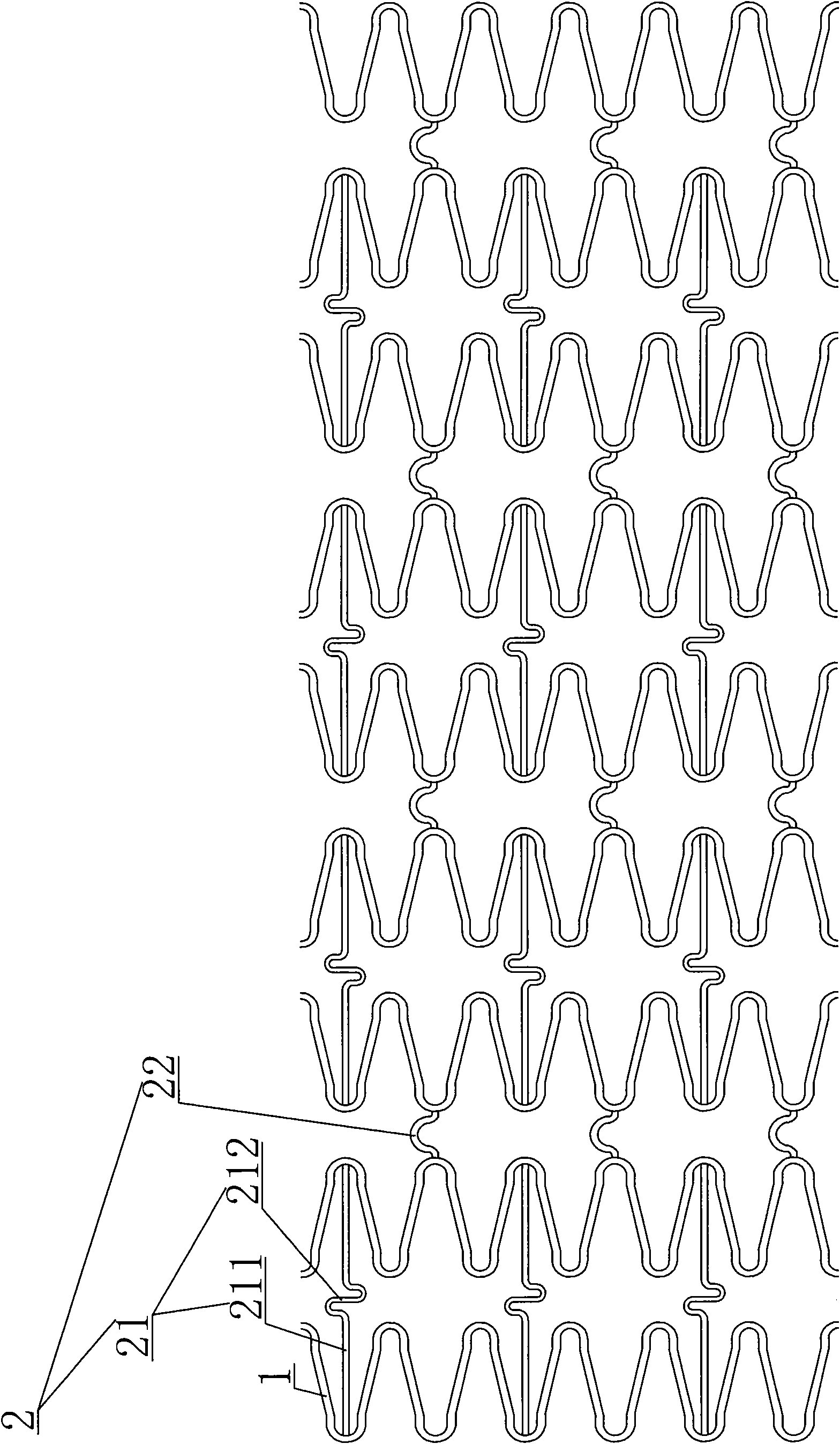Intracranial sirolimus medicament-release blood vessel stent and preparation method thereof
A technology of sirolimus and vascular stent, which is applied in the field of intracranial sirolimus drug-releasing vascular stent and its preparation, can solve the problem of high pressure in the balloon name associated with coronary stent and increase the difficulty of intracranial arterial stenosis angioplasty. It can reduce the incidence of stent shedding, avoid poor axial adherence of blood vessels, and reduce bending pressure.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
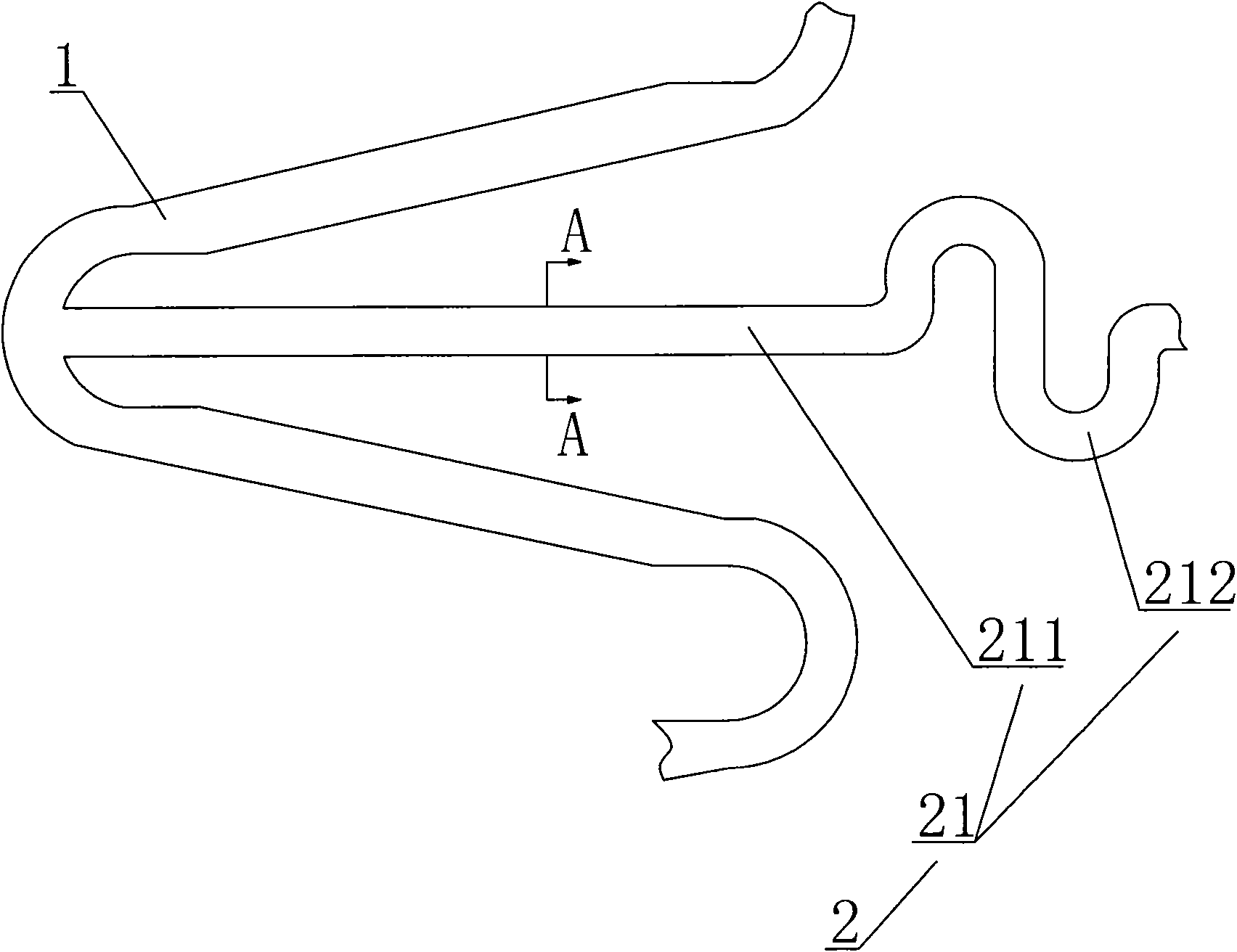
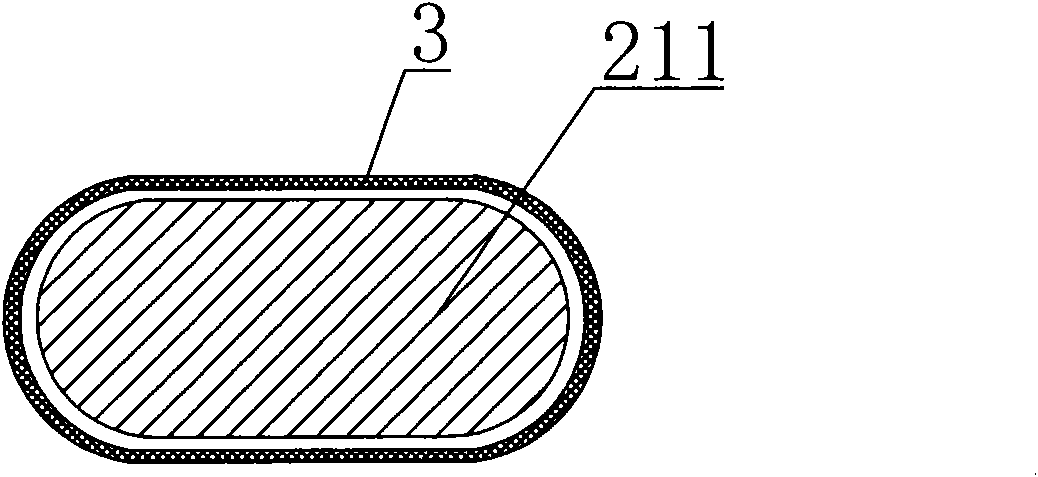
[0032] see Figure 1-3 As shown, an intracranial sirolimus drug-releasing vascular stent is a network-like structure formed of a cobalt-chromium alloy L605 tube through laser engraving and electrochemical polishing; the network-like structure includes multiple sinusoidal waveforms. The supporting rod 1 of the unit structure and the connecting rod 2 connected between the supporting rods 1; the connecting rod 2 includes a long straight line "S" type connecting rod 21 and an arc-shaped connecting rod 22; each of the supporting rods 1 consists of six One has a sinusoidal wave unit structure; the sinusoidal wave unit of the two adjacent support rods 1 adopts a corresponding form in which the trough of one support rod 1 is adjacent to the peak corresponding to the other support rod 1; the peak of one end support rod 1 A long straight line "S"-shaped connecting rod 21 is provided between the troughs corresponding to adjacent support rods 1, and the long straight line "S"-shaped conne...
Embodiment 2
[0050] An intracranial sirolimus drug-releasing vascular stent differs from Embodiment 1 in that each support rod 1 is composed of nine unit structures with sinusoidal waveforms.
[0051] The rib width of the "Ω" curved angle structure of the support rod 1 is 0.0660 mm, the width of the straight structure rib of the support rod 1 is 0.0787 mm, and the rib width of the long straight line "S" type connecting rod is 0.0635 mm.
[0052] The transverse network tube length of the vascular stent is 8mm.
[0053] A preparation method for an intracranial sirolimus drug-releasing vascular stent, the steps are as follows:
[0054] 1. Cobalt-chromium alloy L605 tubing is processed by laser engraving and electrochemical polishing to prepare a vascular stent with a network tubular structure;
[0055] II. Surface pretreatment of the network tubular structure vascular stent: use purified water to soak and clean the network tubular structure vascular support;
[0056] III. Prepare three part...
Embodiment 3
[0063] An intracranial sirolimus drug-releasing vascular stent, which is different from Embodiment 2 in that each support rod 1 is composed of seven unit structures with sinusoidal waveforms.
[0064] The rib width of the "Ω" shaped corner structure of the support rod 1 is 0.0711 mm, the width of the straight structure rib of the support rod 1 is 0.0838 mm, and the rib width of the long straight "S" type connecting rod is 0.0660 mm.
[0065] The transverse network tube length of the vascular stent is 38mm.
[0066] A preparation method for an intracranial sirolimus drug-releasing vascular stent, the steps are as follows:
[0067] 1. Cobalt-chromium alloy L605 tubing is processed by laser engraving and electrochemical polishing to prepare a vascular stent with a network tubular structure;
[0068] II. Pretreatment of the surface of the vascular stent with a network tubular structure: use acetone solvent to soak and clean the vascular stent with a network tubular structure;
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com