Process for production of oxidized cyclic phenol sulfide
A technology of sulfide and oxidation type, applied in the direction of organic chemistry, organic chemistry, etc., can solve the problems of undeveloped preparation methods and industrial application of compounds, and achieve the goal of improving production efficiency, shortening reaction time, and reducing environmental load Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The analysis of the purity and composition ratio of the oxidized cyclic phenol sulfide prepared by the method of the present invention is carried out by high performance liquid chromatography (hereinafter referred to as HPLC), and the structure analysis is carried out by LC / MS measurement. The measurement conditions of HPLC are as follows. Apparatus: LC-10A manufactured by Shimadzu Corporation, Column: Develosil ODS-HG-5 manufactured by Nomura Chemical Co., Ltd. (inner diameter: 4.6 mm, column length: 250 mm), column temperature: 40°C, mobile phase: tetrahydrofuran / acetonitrile / water / trifluoroacetic acid=350 / 350 / 300 / 2 (v / v / v / v), flow rate: 1.0 ml / min, injection volume: 1 μL, sample concentration: 1000 ppm. The measurement conditions of LC / MS are as follows. (1) HPLC measurement conditions, device: Waters Co., Ltd. 2695, column: Shiseido Co., Ltd. Kapselpack C18ACR (5 μm, inner diameter 4.6 mm, column length 250 mm), column temperature: 40°C, mobile Phase: tetrahydrof...
Embodiment 2
[0042] Since repeated reproducibility is required to apply the present invention to industry, a reproducibility confirmation experiment was performed under the same conditions as in Example 1. As a result, 174.6 g (86% yield) of an oxidized cyclic phenol sulfide was obtained.
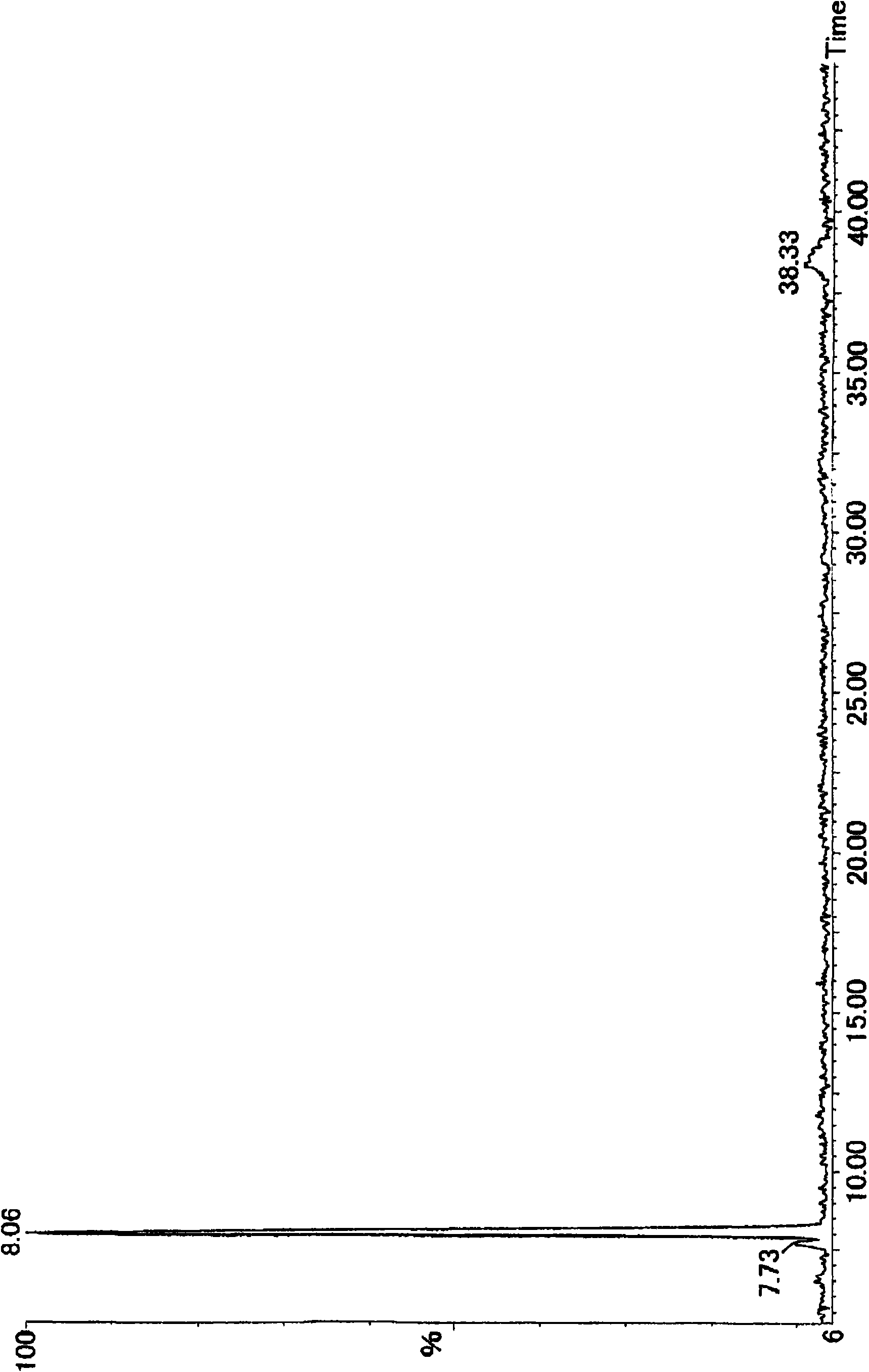
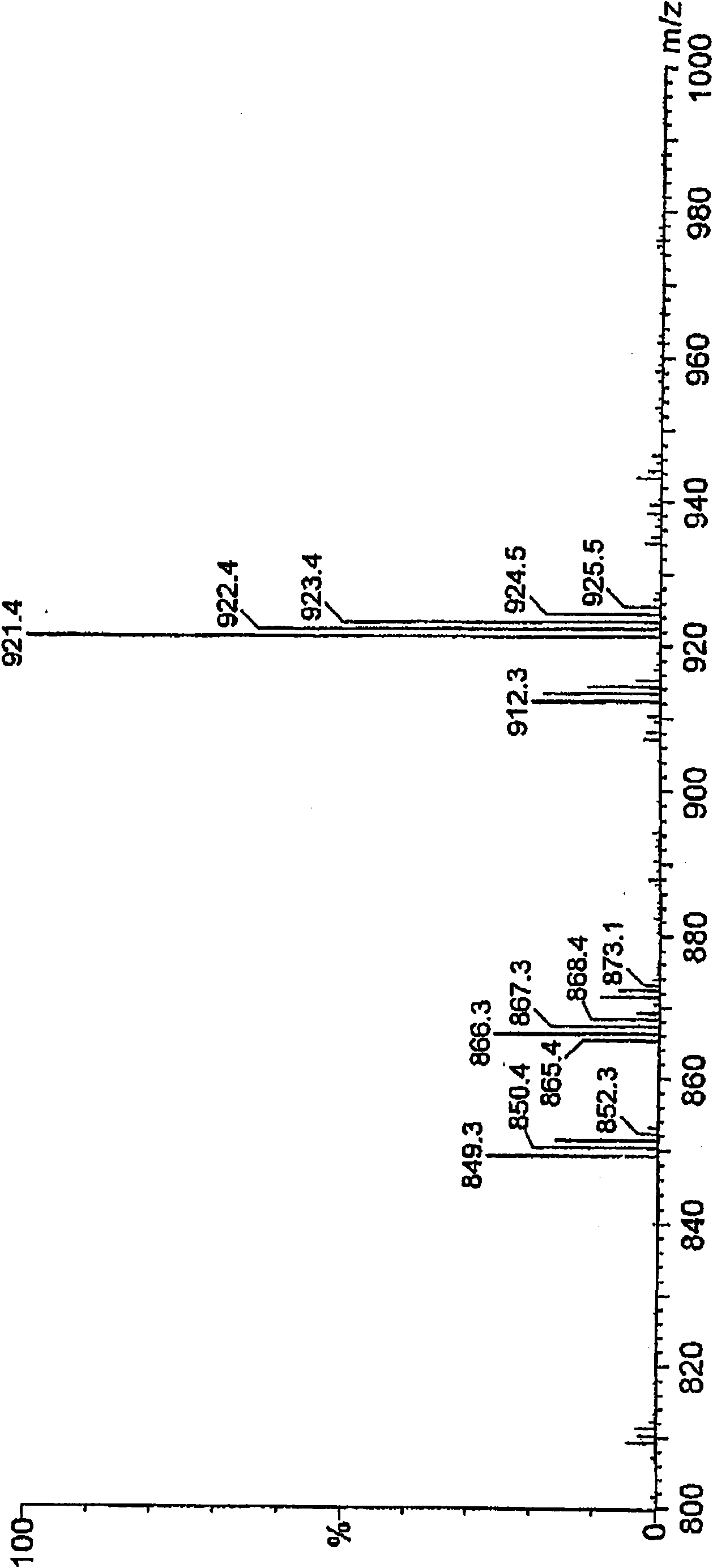
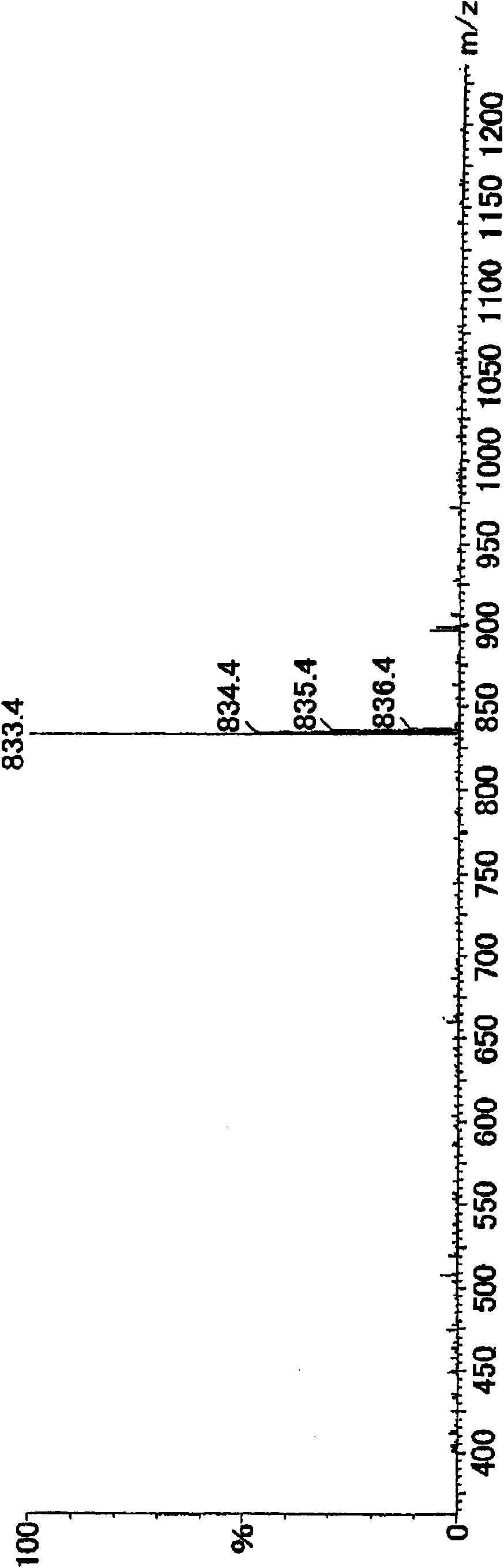
[0043] The obtained oxidized cyclic phenol sulfide was analyzed by LC / MS Figure 5 Total ion chromatogram (TIC) shown. As a result of structural analysis, it was confirmed that oxidized cyclic tetraploid (oxidized cyclic phenol sulfide in general formula (2) where R = tert-butyl, m = 4, and n = 2) and oxidized cyclic octaploid (R = tert-butyl, m = 8, n = 2 oxidized cyclic phenol sulfide in the general formula (2)). The composition ratio determined by HPLC analysis is: the oxidized cyclic tetraploid has a peak area ratio of 90.9%, and the oxidized cyclic octoploid has a peak area ratio of 5.8%.
[0044] In addition, the sodium content in the obtained oxidized cyclic phenol sulfide was 743 ppm.
Embodiment 3
[0046] Under the same conditions as in Example 1, the reproducibility confirmation experiment was carried out on an enlarged scale.
[0047] In a 10L four-necked flask with a stirrer, a cooling tube, and a thermometer, add a cyclic tetraploid in which R is a tert-butyl group and m is 4 in the general formula (1) and R in the general formula (1) is a tert-butyl group, The composition ratio of the cyclic octaploid whose m is 8 is 1298g (1.8 moles) of the above-mentioned cyclic phenol sulfide, 5200g (4 times wt / wt) of acetic acid, and 118.7g (0.36 mol), 122.4 g (0.90 mol) of sodium acetate·trihydrate, and the temperature was raised to 60°C. 2800 g (29 mol) of 35% hydrogen peroxide aqueous solution was added dropwise into the flask through the dropping funnel over about 3 hours.
[0048] After completion of the dropwise addition, the reaction was carried out at 60° C. for 15 hours, then at 70° C. for 2 hours, and then at 80° C. for 3.5 hours. After adding dropwise 187.5 g (1.8 m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com