Method for purifying taxane compound
A technology of taxanes and purification methods, which is applied in the field of anhydrous crystalline taxane compounds, can solve the problems of large amount of resin, high cost, and long operation cycle, and achieve the effects of mild conditions, easy control, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0130] Embodiment 1: the purification of paclitaxel
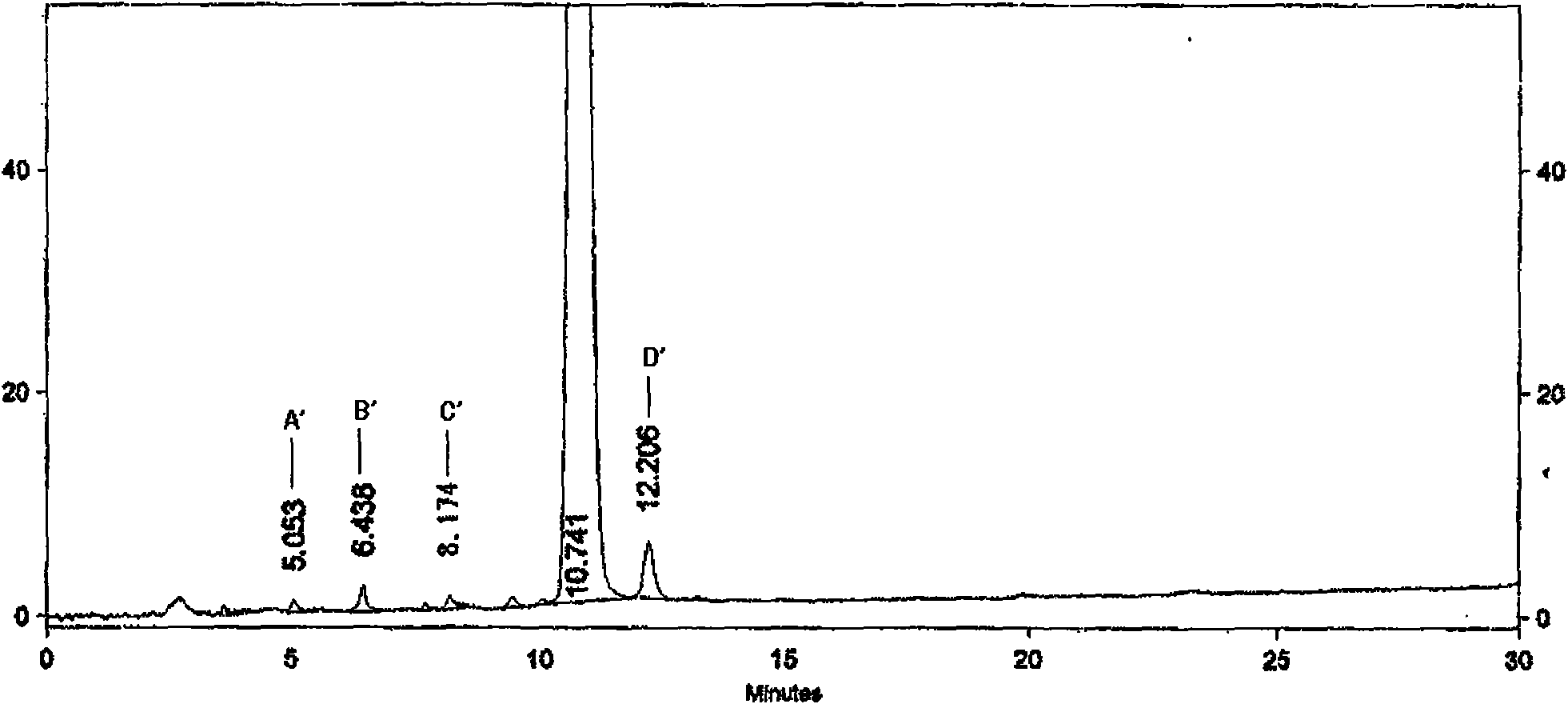
[0131] Impurities in crude paclitaxel:
[0132] For crude paclitaxel, its common impurities are: 10-deacetyl-Paclitaxel of paclitaxel (10-Deacetyl-Paclitaxel), epimer of paclitaxel (7-epi-paclitaxel, 7-Epi-Paclitaxel), the epimer The 10-position deacetylated (7-Epi-10-Deacetyl-Paclitaxel) and cephalomannine (Cephalomannine). The molecular structural formulas of these four impurities are as follows:
[0133]
[0134] Paclitaxel 10-position deacetylate
[0135]
[0136] 7-epitaxol
[0137]
[0138] 7-epitaxol 10 deacetylation
[0139]
[0140] cephalomannine
[0141] Paclitaxel purification process:
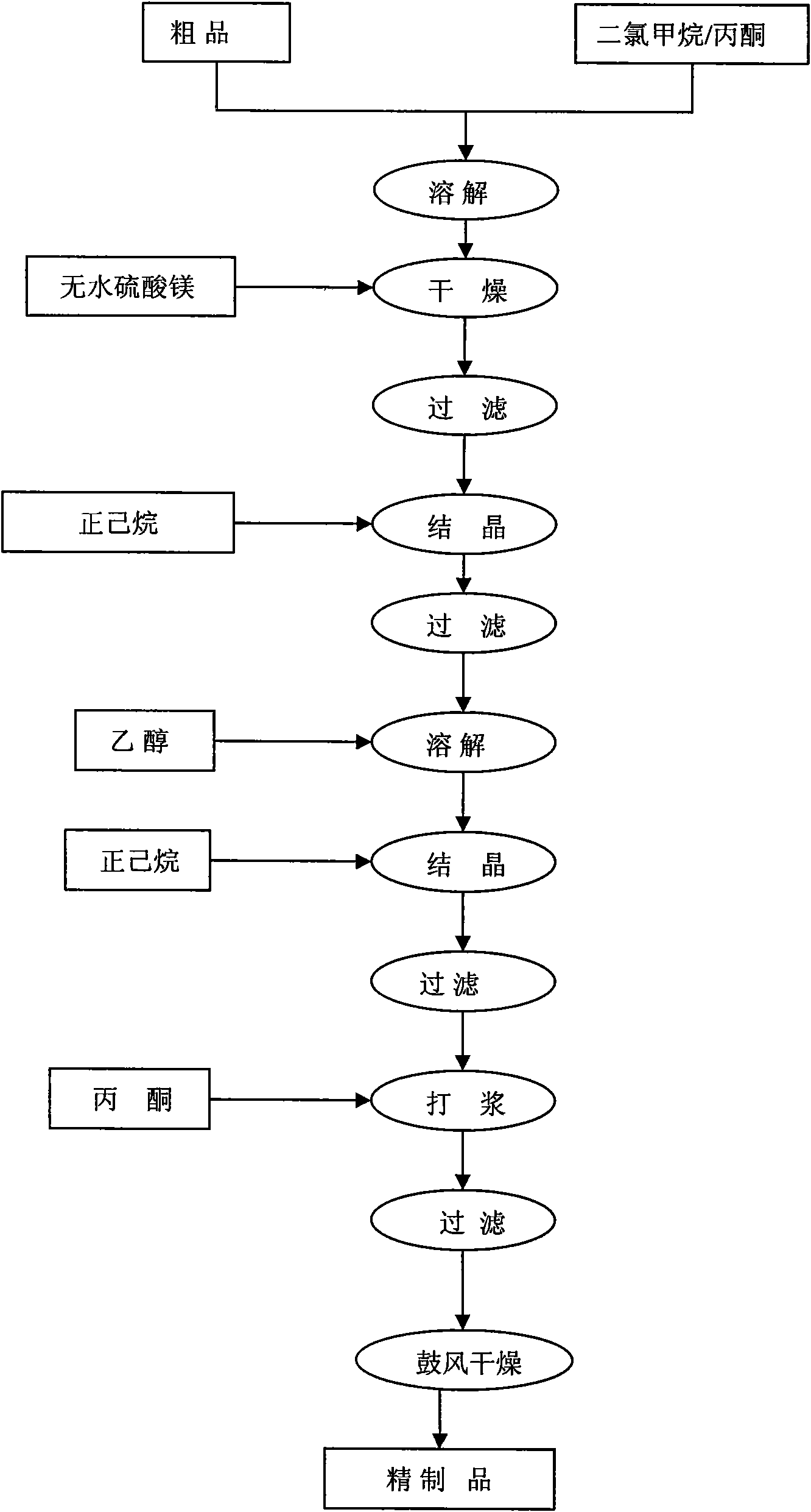
[0142] Primary recrystallization: Add 0.80KG of crude paclitaxel and 12L of chloroform / acetone (v / v=2 / 1) mixed solvent into a 30L reaction kettle at 10°C, and stir to dissolve. The organic phase was dried with 0.8 kg of anhydrous sodium sulfate for 4 hours. Remove sodium sulfate by filtration, control the tem...
Embodiment 2
[0150] Embodiment 2: Purification of Docetaxel
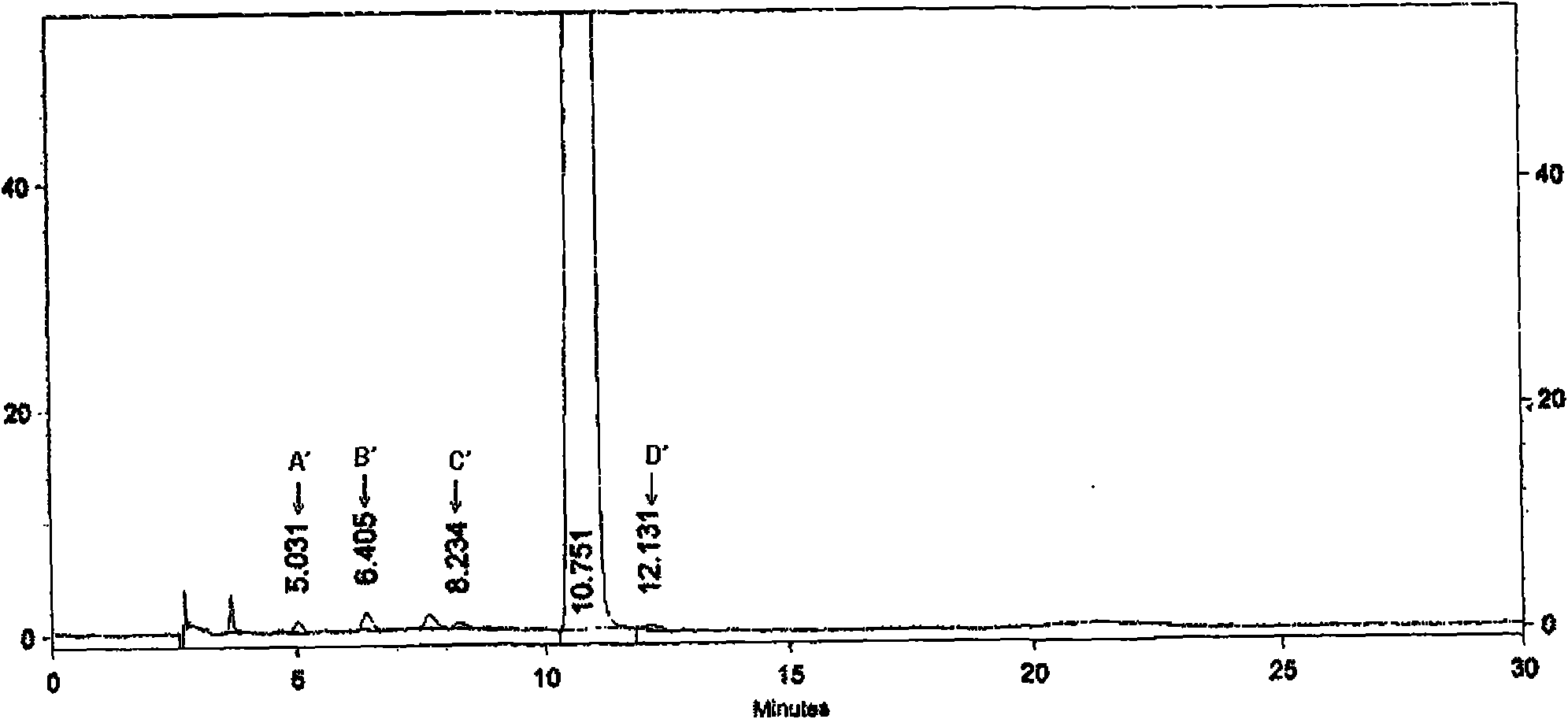
[0151] Docetaxel crude product contains impurities:
[0152] For the crude product of docetaxel, the common impurities are: 10-oxo-docetaxel (10-oxo-docetaxel) of docetaxel, epimers of docetaxel (7-epi-docetaxel, 7-Epi-docetaxel ) and the 10-oxyhydroxide of this epimer (7-Epi-10-oxo-docetaxel). The molecular structural formulas of these three impurities are as follows:
[0153]
[0154] Docetaxel 10-hydroxyl oxide
[0155]
[0156] 7-epidocetaxel
[0157]
[0158] 7-epidocetaxel 10-hydroxyl oxide
[0159] Purification process of docetaxel:
[0160] Primary recrystallization: Add 0.84KG of crude docetaxel and 6.5L of dichloromethane / acetone (v / v=2 / 1) mixed solvent into a 30L reaction kettle at 15°C, and stir to dissolve. The organic phase was dried with 0.7 kg of anhydrous magnesium sulfate for 3 hours. Remove magnesium sulfate by filtration, control the temperature at 5°C, slowly add 15L of n-hexane to crystalliz...
Embodiment 3
[0168] Example 3: Purification of 10-deacetylbaccatin III
[0169] Primary recrystallization: Add 0.2KG 10-deacetylbaccatin III crude product and 2L dichloromethane / acetone (v / v=2 / 1) mixed solvent into a 10L reaction flask at 25°C, stir to dissolve. The organic phase was dried with 0.1 kg of anhydrous magnesium sulfate for 3 hours. Remove magnesium sulfate by filtration, control the temperature at 25°C, slowly add 4L of n-hexane to crystallize under stirring, continue to heat and crystallize for 25 minutes, filter, and dry to obtain a recrystallized product;
[0170] Secondary recrystallization: Add the primary recrystallization product obtained in the previous step and 2L absolute ethanol into a 10L reaction flask at 25°C, and stir to dissolve. Control the temperature at 0°C, slowly add 4L of n-hexane to crystallize under stirring, continue to keep warm for crystallization for 25 minutes, filter, and dry to obtain the secondary recrystallization product;
[0171] Beating: a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com