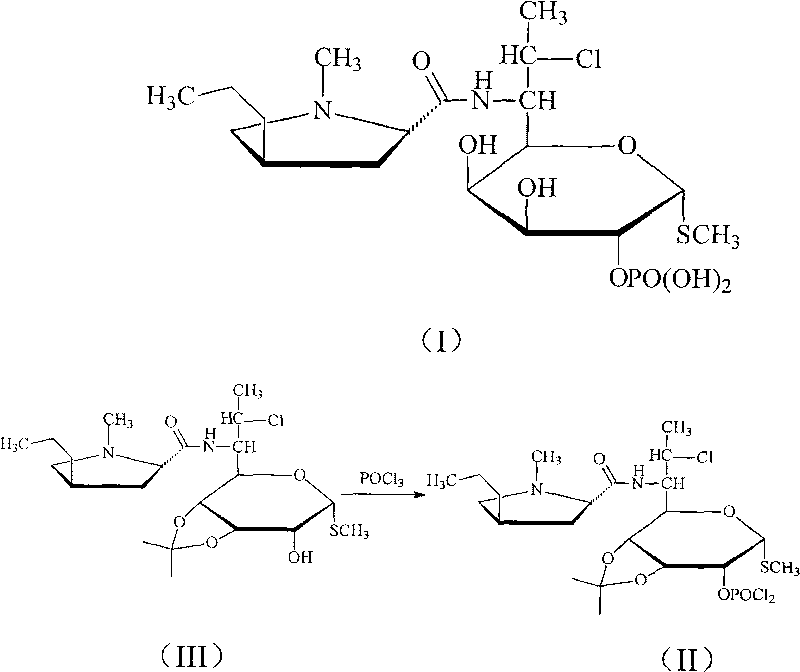
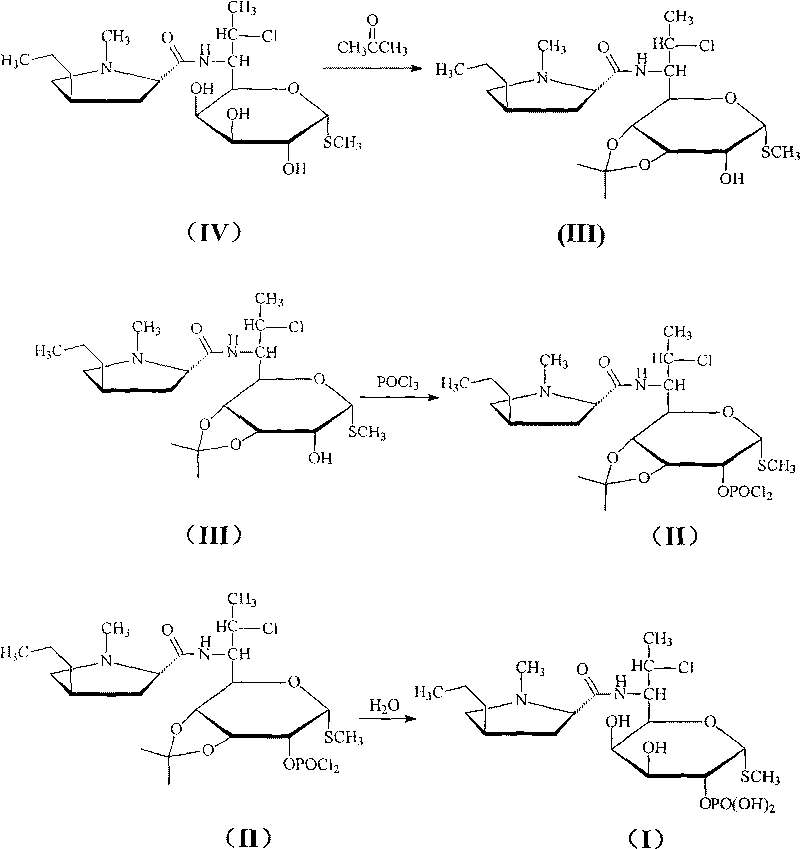
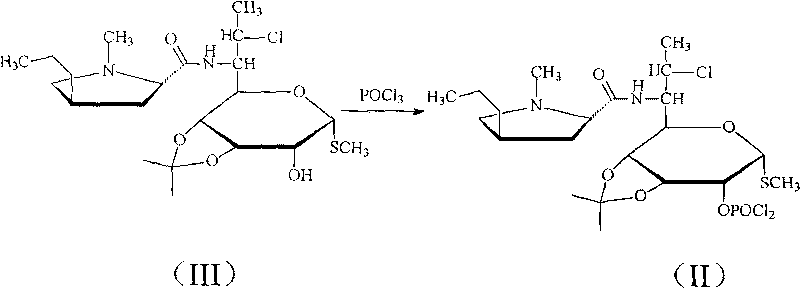
Novel route for clindamycin phosphate compounds
A technology of clindamycin phosphate and clindamycin, which is applied in organic compound/hydride/coordination complex catalysts, chemical/physical processes, sugar derivatives, etc., and can solve the problem that related substances do not meet the requirements of pharmacopoeia standards , high production costs, expensive and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1 3, the synthesis of 4-oxo-isopropylidene clindamycin
[0034] The clindamycin of 100 grams (0.24mol) is joined in the acetone of 1500ml, then add 35 grams (0.26mol) zinc chloride powder, stir and react at room temperature for 12 hours, most of acetone is recovered by distillation under reduced pressure, then add 5 500ml of sodium bicarbonate solution and 500ml of ether, stirred, allowed to stand, layered, the organic phase was washed with 300ml of distilled water, then dried with anhydrous sodium sulfate, distilled under reduced pressure to obtain viscous, recrystallized with ethyl acetate , 99.2 g of the product was obtained, the yield was 89%, and the purity was 99.9%.
Embodiment 2
[0035] Example 2 Synthesis of 3,4-oxo-isopropylidene clindamycin phosphate
[0036] 50 grams (0.11mol) of 3,4-oxo-isopropylidene clindamycin was added to 300ml of carbon tetrachloride, nitrogen protection was introduced, and then N,N-diisopropylethylamine 100ml was added and N, N-dimethylaminopyridine 5 grams, the mixture was cooled to 0 ° C, slowly added dropwise 23 grams of phosphorus oxychloride, to maintain the reaction temperature is not more than 5 ° C, then reacted at 0 ° C for 1 hour, standing, The layers were separated, and the organic phase was washed with 200 ml of water, dried over anhydrous sodium sulfate, and concentrated under reduced pressure at 50°C to obtain 54.5 g of the product, with a yield of 92% and a purity of 99.7%.
Embodiment 3
[0037] Example 3 Synthesis of Clindamycin Phosphate
[0038] Add 40 grams of 3,4-oxo-isopropylidene clindamycin phosphate into 200ml of acetic acid and 40ml of water, then heat to 85°C, react for 1.5 hours, then distill off most of the acetic acid under reduced pressure, and then add 100ml of water and 300ml of absolute ethanol were cooled to 5°C, stirred, and solids were precipitated, filtered, washed with ethanol, and dried at 60°C to obtain 33.3 g of the product, with a yield of 90%, a purity of 99.7%, and Mp: 209°C.
[0039] Elemental Analysis C 18 h 34 ClN2 o 8 PS molecular weight: 504.97
[0040] Theoretical value C: 42.8%, H: 6.8%, N: 5.5%, O: 25.4%, P: 6.1%, Cl: 7.0%, S: 6.4%;
[0041] Found values C: 42.7%, H: 6.9%, N: 5.6%, O: 25.3%, P: 6.0%, Cl: 7.2%, S: 6.3%
[0042] 1 HNMR (CD 3 OD) δ7.2-7.45 (5H, m, Ph-H), 5.47 (1H, d, CONH), 5.11 (2H, m, ArCH 2 O), 4.1-4.8 (5H, m, 50CH - ), 3.89 (1H, m, R-CHCl), 3.76 (2H, m, 2NCH - ), 2.2-2.5 (3H, m, N-CH 3 ), 2.18 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com