Ethylene-propylene-diene methylene using liquid-state oligomers of diolefin as third monomer and method for preparing same
A technology of EPDM rubber and oligomer, applied in the field of EPDM rubber and its preparation, can solve the problems of high price, slow vulcanization, easy explosion, etc., and achieves good processing performance, good copolymerization ability, Beneficial to industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] In the 0 and 5L reactors fully replaced with nitrogen, respectively add: (1) 300ml hexane; (2) 0.10mmol vanadium catalyst; (3) 3.0mmol trichlorotriethyldialuminum, molecular formula: Al 2 (C 2 h 5 )3 Cl 3 ; 0.15g diene liquid oligomer A, 1,2-structure content at 20%; (4) ethyl trichloroacetate of 0.60mmol, (hereinafter referred to as: ETCA); (5) pass into ethylene and propylene mixed gas , the molar ratio of ethylene and propylene in the mixed gas is 1:2, the pressure reaches 4MPa, stir, and react at 20°C for 15min.
[0029] After the polymerization, 5 ml of 5% by mass hydrochloric acid-ethanol solution was added to the polymer product, washed with ethanol, and then dried under vacuum to obtain 12.2 g of polymer.
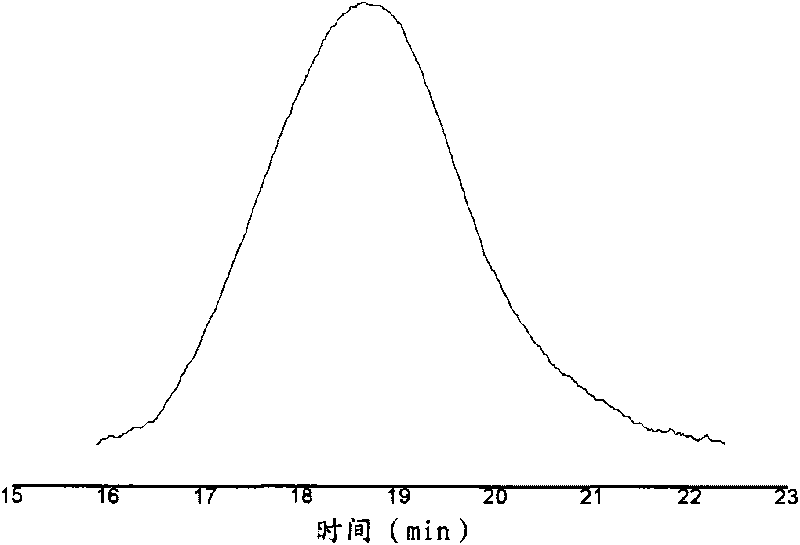
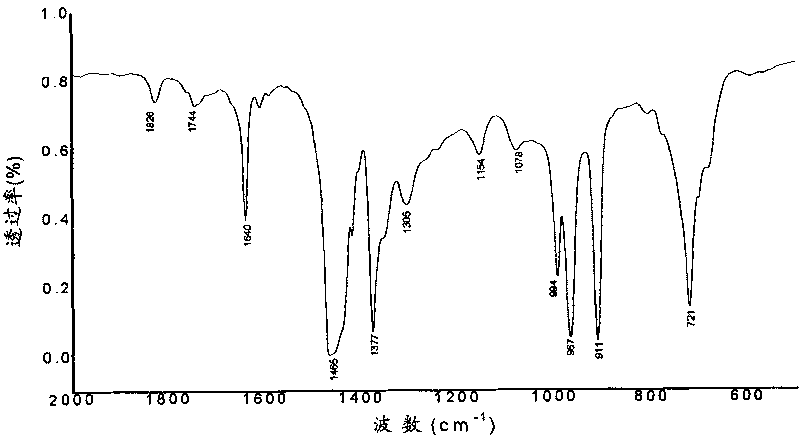
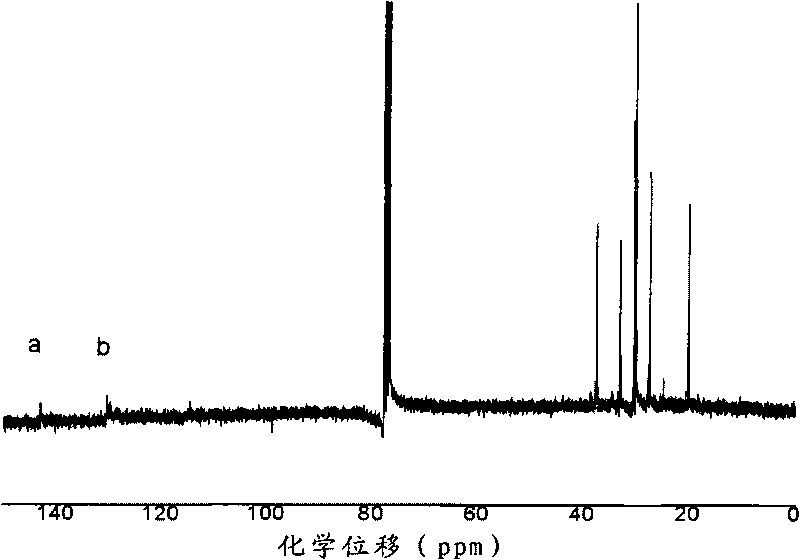
[0030] Through differential calorimetry (DSC) analysis, the glass transition temperature of gained polymer is 55.6 ℃; 13 C-NMR spectral analysis, the combined propylene molar content (C 3 mol%): 34%, double bond content: 1.2%; GPC analysis shows that the...
Embodiment 2-6
[0032] Use 1.0g, 2.5, 3.7g, 4.5g, 6g of diene liquid oligomer A respectively, and the polymerization conditions are: (1) 0.5L reactor; (2) Vanadium-based catalyst: 0.10mmol; (3) Al 2 (C 2 h 5 ) 3 Cl 3 : 3.0mmol, diolefin liquid oligomer A, 1,2-structure content at 20%; (4) ETCA: 0.60mmol; (5) 300ml solvent hexane; (6) ethylene, propylene molar ratio in the mixed gas is 1:2, pressure: 4MPa, react at 20°C for 15min.
[0033] The rest carry out polymerization reaction and polymer analysis test with [embodiment 1] same method and condition. The results are listed in Table 1.
[0034] Table 1
[0035] no
Embodiment 7-11
[0037] Respectively use 2.5g diene liquid oligomer A, 1,2-structure content at 30%, 40%, 50%, 65%, 80% substitute [embodiment 1] in the diene liquid oligomer A of 0.15g , 1,2-structure content at 20%, the polymerization conditions are: (1) 0.5L reactor; (2) vanadium series catalyst: 0.10mmol; (3) Al 2 (C 2 h 5 ) 3 Cl 3 : 3.0mmol, diene liquid oligomer A: 2.5g; (4) ETCA: 0.60mmol; (5) 300ml solvent hexane; (6) the molar ratio of ethylene and propylene in the mixed gas is 1: 2, pressure: 4MPa , reacted at 20°C for 15min.
[0038] The rest carry out polymerization reaction and polymer analysis test with [embodiment 1] same method and condition. The results are listed in Table 2.
[0039] Table 2
[0040] no
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com