Phosphoric acid diester and preparation method thereof
A technology of phosphodiester and cyanoborane phosphate, which is applied in the field of phosphodiester and its preparation, can solve the problems that have not yet been achieved at the same time, and achieve the effects of good nuclease hydrolysis and large membrane permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
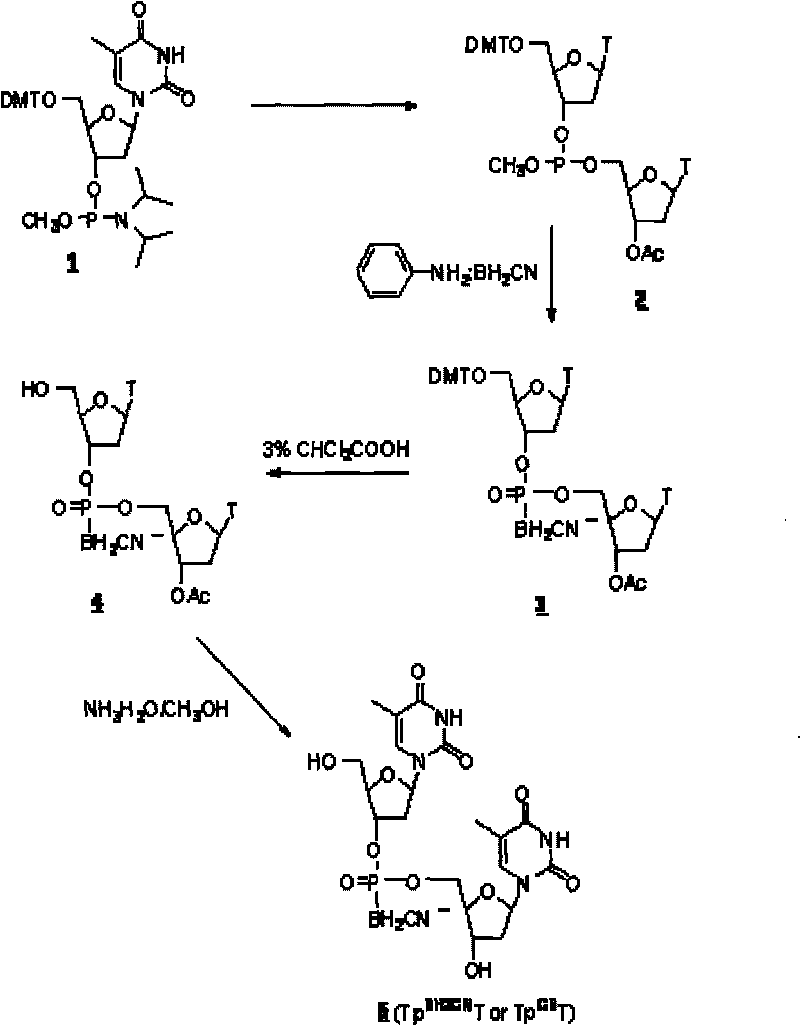
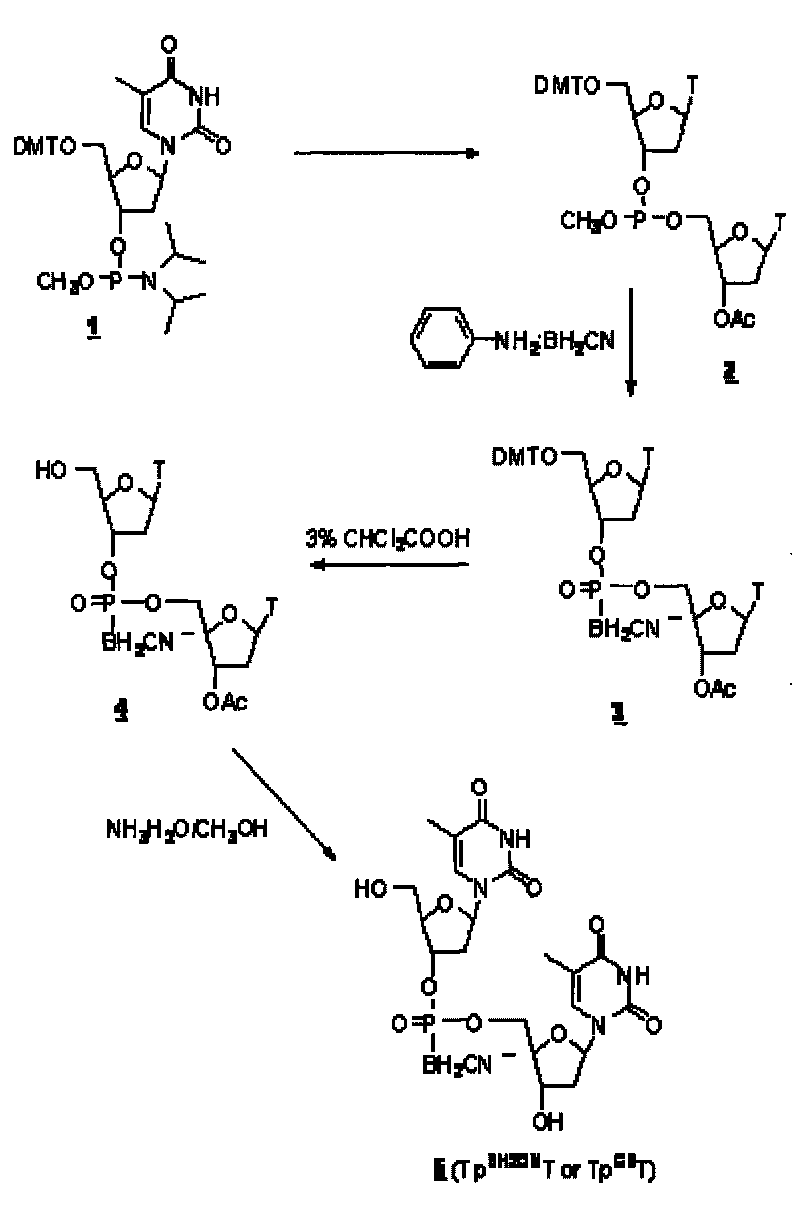
[0043] DNA cyanoboranyl phosphate analog: double thymidine cyanoboranyl phosphate (Tp BH2CN T or Tp CB T) synthesis:
[0044] Dinucleoside cyanoborane phosphate (Tp BH2CN T or Tp CB The synthetic route of T) is as figure 1 shown.
[0045] a. Nucleoside coupling:
[0046] At room temperature, protected thymidine phosphoramidites ( 1 ) (0.5mmol, purchased from U.S. Chemgenes) reacted with acetylthymidine (0.5mmol) in dimethylformamide DMF (3ml) for 1 hour under the catalysis of tetrazolium (0.5mmol), to obtain phosphorous acid ester( 2 ).
[0047] b, cyanoborane substitution:
[0048] At 68°C, phosphite ( 2 ) reacted with aniline-cyanoborane complex (4mmol) in tetrahydrofuran (12ml) for 2 hours to obtain phosphite-cyanoborane ( 3 ).
[0049] C. Deprotection:
[0050] The compound ( 3 ) After half an hour, the protected dinucleoside cyanoborane phosphate ( 4 ). After removing the solvent with low boiling point by rotary evaporation under reduced pressure, the compo...
Embodiment 2
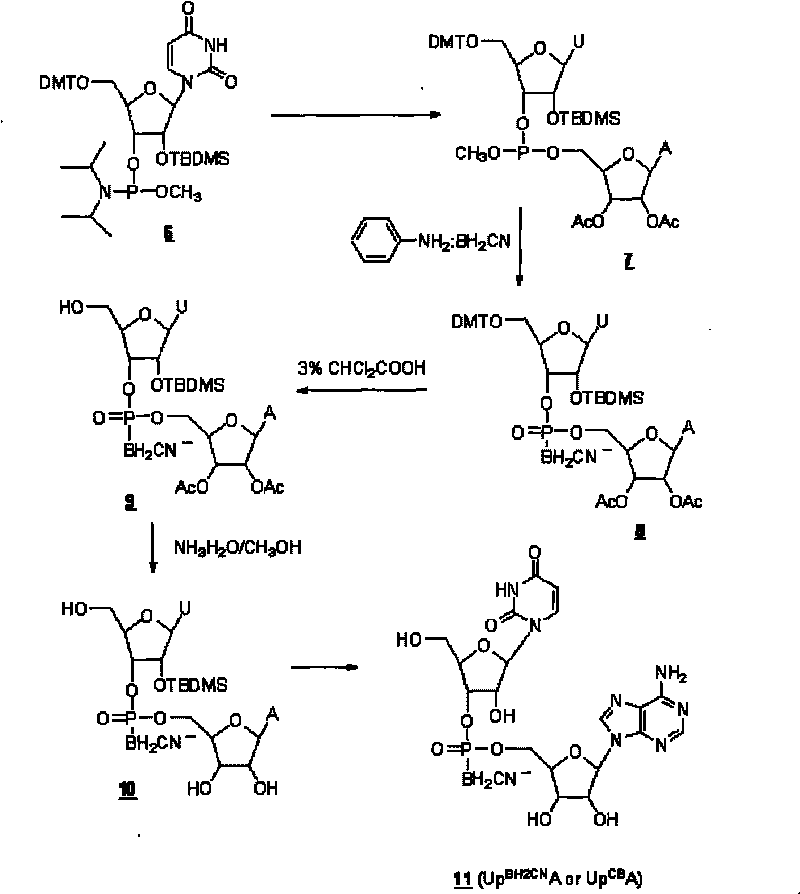
[0057] RNA cyanoboranyl phosphate analogue: Uridine adenine cyanoboranyl phosphate (Up BH2CN A or Up CB A) Synthesis.
[0058] Uridine adenine cyanoborane phosphate (Up BH2CN A or Up CB A) the synthetic route such as Figure II shown.
[0059] a. Nucleoside coupling:
[0060] At room temperature, the protected uridine phosphoramidite ( 6 ) (0.5mmol, purchased from U.S. Chemgenes Company) reacted with diacetyl adenine nucleoside (0.5mmol) in dimethylformamide DMF (6ml) for 2 hours under the catalysis of tetrazolium (0.5mmol), to obtain Phosphite ( 7 ).
[0061] b, cyanoborane substitution:
[0062] At 68°C, phosphite ( 7 ) reacted with aniline-cyanoborane (4mmol) in tetrahydrofuran (16ml) for 3 hours to obtain phosphite-cyanoborane ( 8 ).
[0063] c. Deprotection:
[0064] Phosphite-cyanoborane ( 8 ) After 1 hour, the protected dinucleoside cyanoborane phosphate ( 9 ). After removing the low-boiling solvent by rotary evaporation under reduced pressure, the protec...
Embodiment 3
[0071] Basic properties of cyanoborane phosphates.
[0072] 1. Acid-base hydrolysis stability.
[0073] Cyanoborane phosphate is very stable in acid-base hydrolysis. At 37° C., under the condition of pH=3 or 11, 100 mM acetic acid / ammonia water was used to treat double thymidine cyanoborane phosphate ( 5 ) for 24 hours, no hydrolysis or degradation products were detected by high performance liquid chromatography. High performance liquid chromatography conditions: 80% 100 mM triethylammonium acetate (Triethylammonium Acetate, TEAA) and 20% acetonitrile as the eluent; the elution flow rate is 1 ml / min.
[0074] 2. Resistance to nuclease hydrolysis.
[0075] The cyanoborane phosphate linkage is very stable to hydrolysis by snake venom phosphodiesterase (SVPDE) and bovine spleen phosphodiesterase (BSPDE). The process of resistance to nuclease hydrolysis was monitored by high performance liquid chromatography. High performance liquid chromatography conditions: the eluent is 80...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com