Aliphatic-aromatic polyesters, and articles made therefrom
一种芳族、制品的技术,应用在聚酯涂料、合成树脂层状产品、单组分共聚酯人造长丝等方向,能够解决过程复杂化等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0167] testing method
[0168] Unless otherwise stated, the following test methods were used in the Examples and Comparative Examples disclosed herein.
[0169] Differential Scanning Calorimetry (DSC) was performed on a model 2920 TA Instruments machine. Under a nitrogen atmosphere, the sample was heated to 300°C at a rate of 20°C / min, programmed to cool to room temperature at a rate of 20°C / min, and then reheated to 300°C at a rate of 20°C / min. The observed sample T provided below in this paper g and crystal melting temperature (T m ) from the second heating step.
[0170] As used herein, intrinsic viscosity (IV) is as defined by W.R. Sorenson and T.W. Campbell, 1961, p. 35 of "Preparative Methods of Polymer Chemistry". It is determined by the Goodyear R-103B method at room temperature in a 50:50 wt% trifluoroacetic acid:dichloromethane acid solvent system at a concentration of 0.5 g / 100 mL.
[0171] Laboratory Relative Viscosity (LRV) is the ratio of the viscosity of a...
Embodiment 1
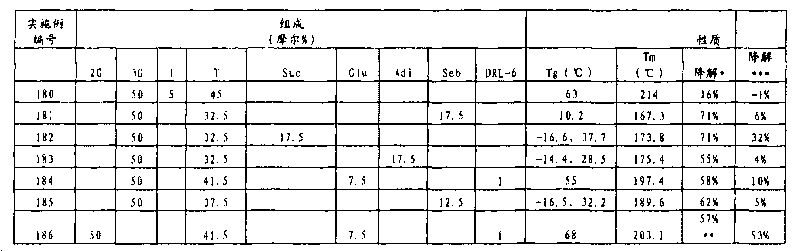
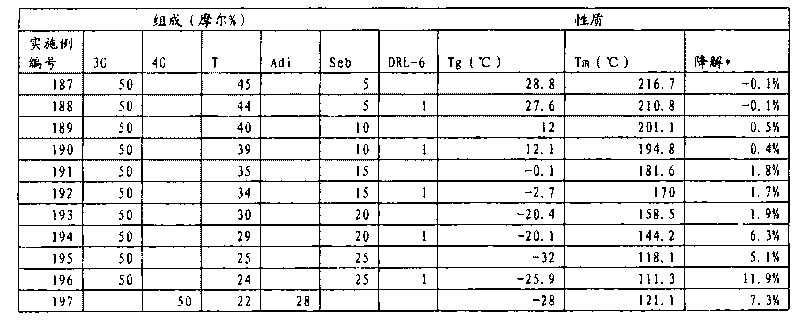
[0178] Into a 250 ml glass flask were charged the following reaction mixture components: dimethyl terephthalate (48.54 g), 1,3-propanediol (38.04 g), sodium isophthalic acid-3-sulfonate (2.96 g) , dimethyl adipate (43.55 g), 1,2,4,5-benzenetetracarboxylic dianhydride (0.098 g), and isopropoxytitanium (IV) (0.0582 g). The reaction mixture was stirred and heated to 180°C under a slow nitrogen purge. After reaching 180°C, the reaction mixture was heated to 200°C over 1.5 hours under a slow nitrogen purge while stirring. The resulting reaction mixture was stirred at 200 °C for 1.0 h while sparging with a slight nitrogen gas. The reaction mixture was then heated to 255°C over 1.0 hour with stirring and a slow nitrogen purge. The resulting reaction mixture was stirred at 255 °C for 0.5 h while sparging with a slight nitrogen gas. 21.35 g of colorless distillate was collected during this heating cycle. The reaction mixture was then placed under full vacuum at 255°C while stirring...
Embodiment 2
[0182] The following reaction mixture components were added to a 1 liter glass flask: dimethyl terephthalate (239.10 g), 1,3-propanediol (247.33 g), dimethyl isophthalate-3-sodium sulfonate ( 5.55 g), dimethyl succinate (182.68 g), manganese (II) acetate tetrahydrate (0.209 g), and antimony (III) trioxide (0.168 g). The reaction mixture was stirred and heated to 180°C under a slow nitrogen purge. After reaching 180°C, the reaction mixture was heated to 255°C over 3.0 hours under a slow nitrogen purge while stirring. The resulting reaction mixture was stirred at 255°C for 1.6 hours while sparging with a slight nitrogen gas. 164.9 g of colorless distillate was collected during this heating cycle. The reaction mixture was then placed under full vacuum at 255°C while stirring. The resulting reaction mixture was stirred under full vacuum (pressure less than 100 mTorr) for 3.3 hours. The vacuum was then relieved with nitrogen and the reaction mass was allowed to cool to room tem...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com