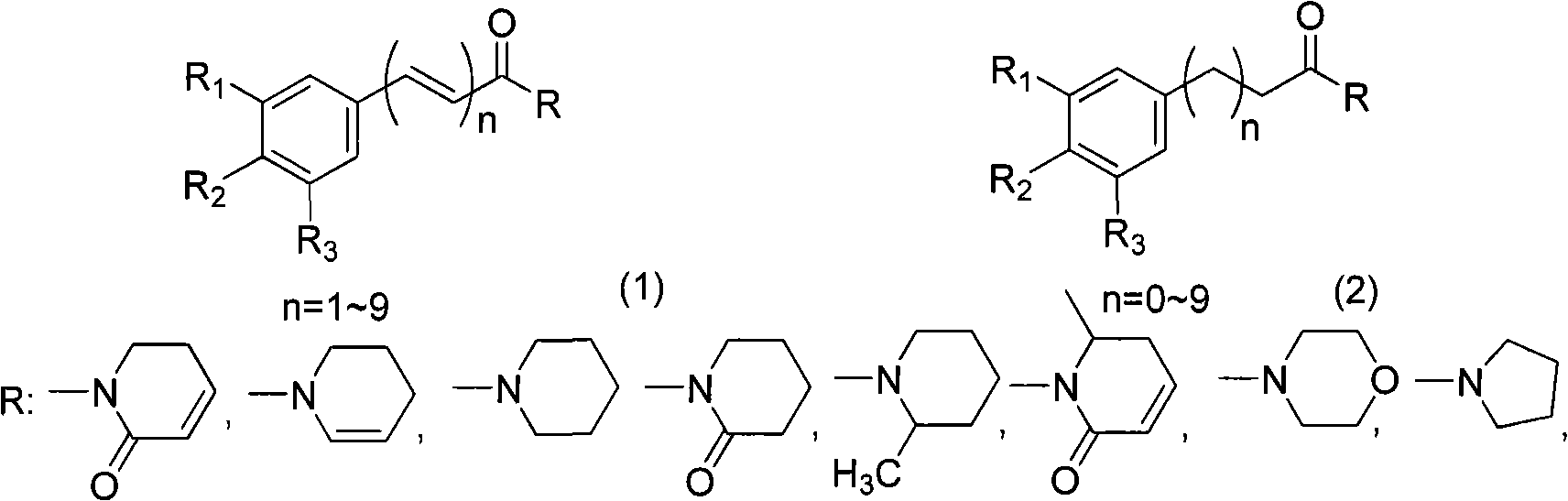
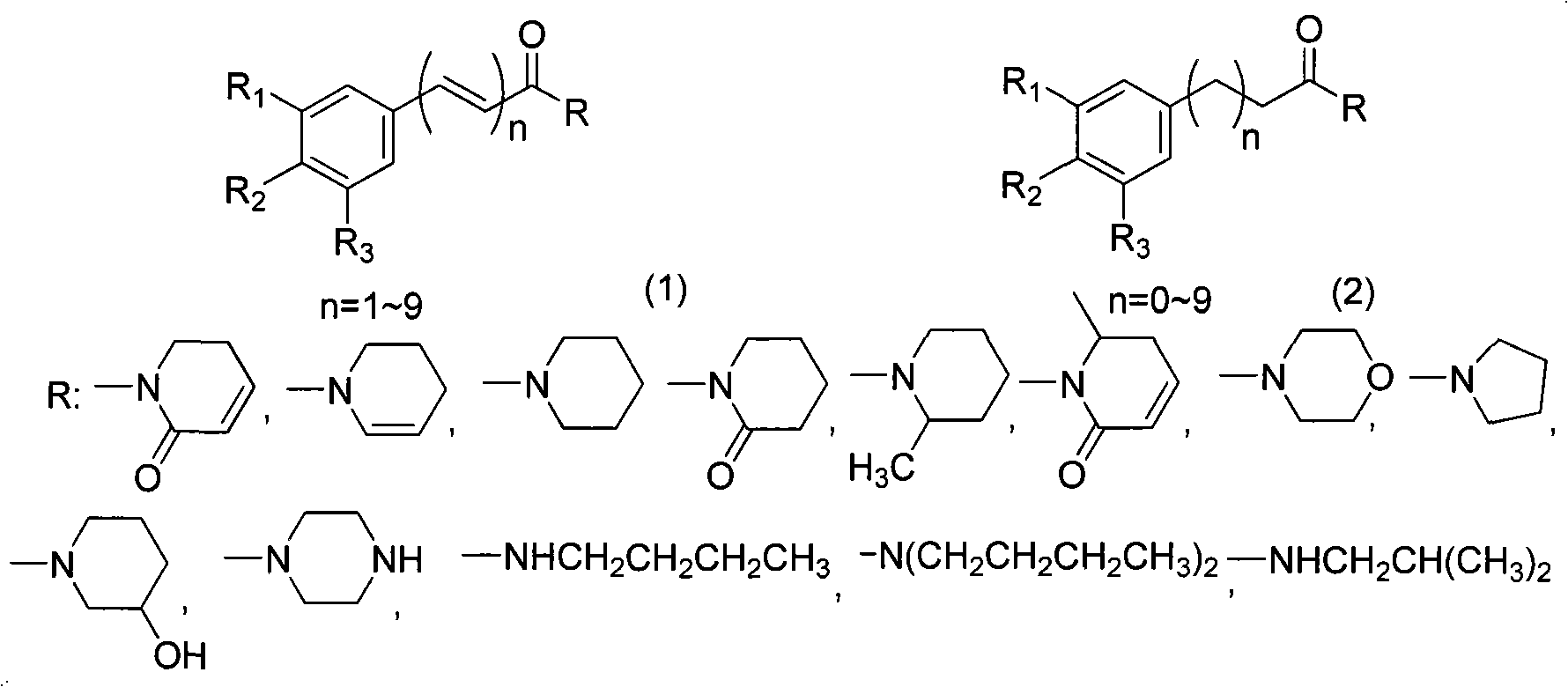
Method for synthesizing piperlongumine compounds
A technology of Piperamide and synthesis method, which is applied in the field of synthesis of alkaloid compounds, can solve the problems of time-consuming plant extraction, compound separation, laborious input-output ratio, etc., and achieves low raw material prices, mild reaction conditions, and easy operation methods. easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
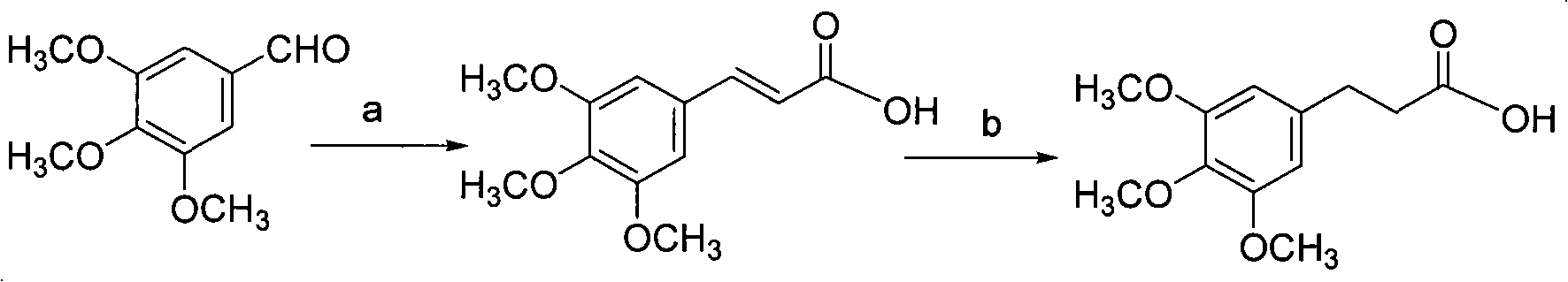
[0027] The synthesis of embodiment 1 aromatic carboxylic acid:
[0028]
[0029] Reagents and conditions: a) propionic acid, pyridine, piperidine; b) H 2 , Pd / C, ethanol.
[0030] 3,4,5,-Synthesis of trimethoxycinnamic acid: 3,4,5,-trimethoxybenzaldehyde 39.2g (0.2mol) and malonic acid 41.6g (0.4mol), dissolved in 300ml pyridine, And add 2.5ml of piperidine, heat to 50°C, react for 3h, until the raw materials are completely reacted, cool to room temperature, add 2M hydrochloric acid to adjust the solution to pH=5, extract with ethyl acetate (200ml×3), combine the organic phases, and separate Washed with saturated brine and secondary water, anhydrous Na 2 SO 4 Dry, concentrate to get crude product, crude product with CH 3 OH·H 2 O was recrystallized to obtain 34.7 g of colorless needle crystals, with a yield of 72%.
[0031] 1 H-NMR: (400MHz, CDCl 3 , ppm) δ: 3.93 (s, 9H), 6.37 (d, 1H), 6.82 (s, 2H), 7.79 (d, 1H), .11.3 (b, 1H) MS-ESI (m / z): 239 (M+H) + .
[0032]...
Embodiment 2
[0034] The synthesis of embodiment 2 amino compounds:
[0035]
[0036] Synthesis of 5,6-dihydro-2(1)-pyridone: 20g vinyl acrylic acid ((E)-penta-2,4-dienoic acid) (0.051mol) and 100ml ammonia water (0°C saturated) were placed in In a 500ml autoclave, heat to 180°C, react for 24h, cool to room temperature, concentrate the reaction solution to obtain a syrupy viscous liquid, add hot methanol, and reflux for 3h, cool to room temperature, concentrate, remove the reaction solvent methanol, reduce Pressure distillation, collection 120 ~ 125 ℃ / 1mmHg, to obtain the product 6g. The target compound is unstable, so it is best to use it now.
[0037] 1 H-NMR: (400MHz, CDCl 3 , ppm) δ: 2.31 (m, 2H), 3.43 (t, 2H), 6.23 (m, 2H), .7.8 (b, 1H, NH), MS-EI (m / z): 97 (M) + .
[0038] The synthesis of 6-methyl-5,6-dihydro-2(1)-pyridone: 20g sorbic acid (2E, 4E)-hexa-2,4-dienoic acid (0.051mol) and 100ml ammonia water (0 ℃ saturated) in a 500ml autoclave, heated to 180°C, reacted for 24...
Embodiment 3
[0040] The synthesis of embodiment 3 Piperamide (compound 1):
[0041]
[0042] 3,4,5-trimethoxycinnamic acid (0.714g, 3mmol) was dissolved in 25ml of dry dichloromethane, 5,6-dihydro-2(1)-pyridone (0.35g, 3.6mmol) was added, and the reaction The system was cooled to -20°C, DCC (0.68 g, 3.3 mmol) was added, the reaction system was gradually warmed to room temperature, and the reaction was continued overnight.
[0043] After the reaction is complete, the reaction system is respectively washed with saturated NaHCO 3 The solution was washed with water twice, the organic phase was dried with magnesium sulfate, concentrated, and the crude product was separated on a silica gel column (eluent: petroleum ether / ethyl acetate=1 / 1) to obtain 0.6 g of Piperamide, yield: 63%.
[0044] 1 H-NMR: (400MHz, CDCl 3 , ppm) δ: 2.45(m, 2H), 3.92(s, 9H), 4.05(m, 2H), 6.12(m, 1H), 6.80(s, 2H), 6.94(m, 1H), 7.37(m , 1H), 7.75 (m, 1H). MS-EI (m / z): 317 (M) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com