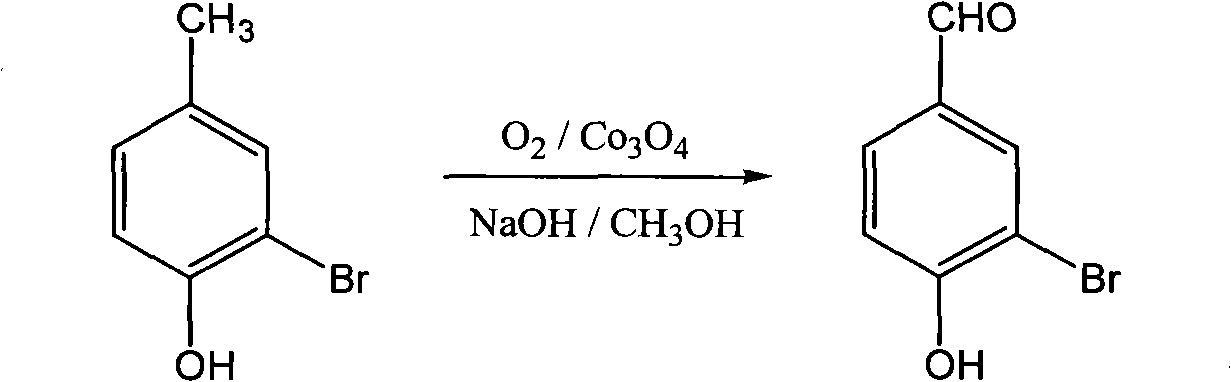Method for preparing vanillin and analogue thereof
A technology of analogs and vanillin, which is applied in the field of preparation of vanillin and its analogs, can solve the problems of expensive raw materials and achieve the effects of increased selectivity, high yield, and high oxidation selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] (1) Synthetic monobromoaldehyde method A
[0025]
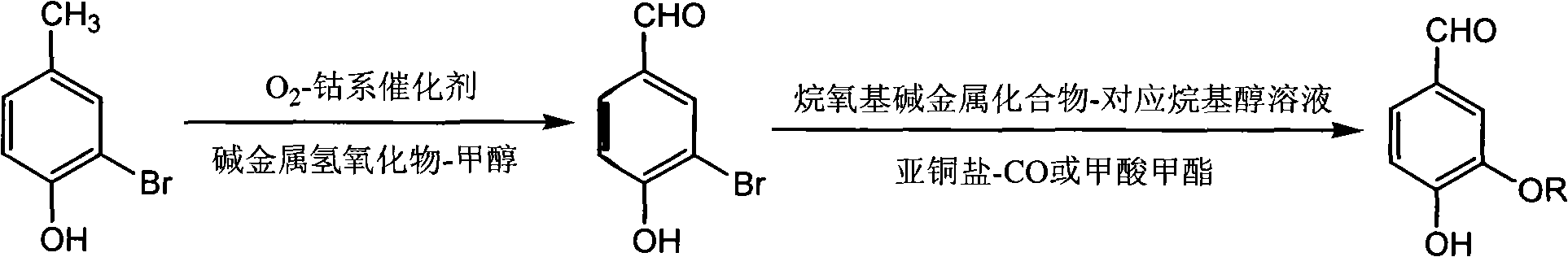
[0026] Add 37.4g (0.20mol) of monobromophenol, 24.0g (0.60mol) of NaOH, 150mL of methanol and 0.96g (0.004mol) of tricobalt tetroxide in the autoclave; Feed oxygen to maintain the pressure to react at 0.6Mpa, and stop the reaction after continuing the oxidation reaction for 6 hours.
[0027] After the reaction was complete, the reactant was transferred to a flask. Recover methanol by distillation. Add 240mL of water, heat to 95°C to completely dissolve monobromoaldehyde phenol sodium salt, remove the catalyst by hot filtration; filter the mother liquor to cool to room temperature, acidify with concentrated hydrochloric acid to pH = 5, and precipitate a large amount of yellow solid; freeze to 0°C to promote its complete Crystallize, filter, wash with water until neutral, and dry in vacuo to obtain 36.2 g of monobromaldehyde yellow powder solid, yield 90.0%.
[0028] Spectral data:
[0029] ESI-MS(m / z): 201(M+1), ...
Embodiment 2
[0039] (1) Synthetic monobromoaldehyde method B
[0040]
[0041] Add 37.4g (0.20mol) of monobromophenol, 24.0g (0.60mol) of NaOH, 150mL methanol and 0.95g (0.004mol) of cobalt chloride hexahydrate in the autoclave; heat after sealing the autoclave, stirring , feed oxygen at 100°C to maintain the pressure at 0.6Mpa, and continue the oxidation reaction for 6 hours before stopping the reaction.
[0042] After the reaction is over, transfer the reactant to a flask, distill and recover methanol, add 240mL of water, heat to 95°C to dissolve the monobromaldehyde phenol sodium salt, heat filter to remove the catalyst; filter the mother liquor to cool to room temperature, acidify to pH with concentrated hydrochloric acid = 5, a large amount of yellow solid precipitated; freeze to 0°C to promote its complete crystallization, filter, wash with water until neutral, and dry in vacuo to obtain 24.5 g of monobromaldehyde yellow powder solid, yield 80.6%.
[0043] Spectral data:
[0044...
Embodiment 3
[0054] Co(NO 3 ) 2 Instead of Co in Example 1 (one) 3 o 4 , other processes are with embodiment 1 (one).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com