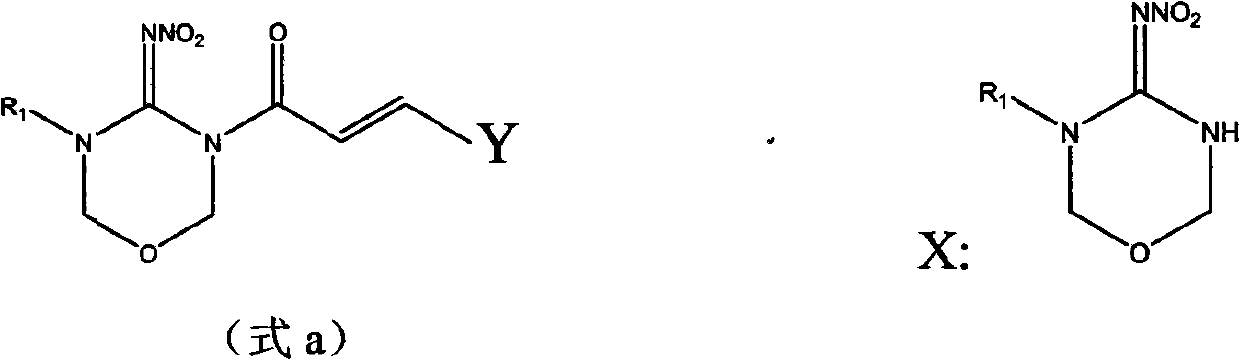Class of aryl-acrylketone compound containing 1,3,5-dioxazine heterocycle, preparation method and application thereof
A technology of aryl acryl ketone and aryl acryloyl chloride, which is applied in the field of aryl acryl ketone compounds, can solve problems such as unclear active ingredients, and achieve the effect of simple product purification method, obvious biological activity, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
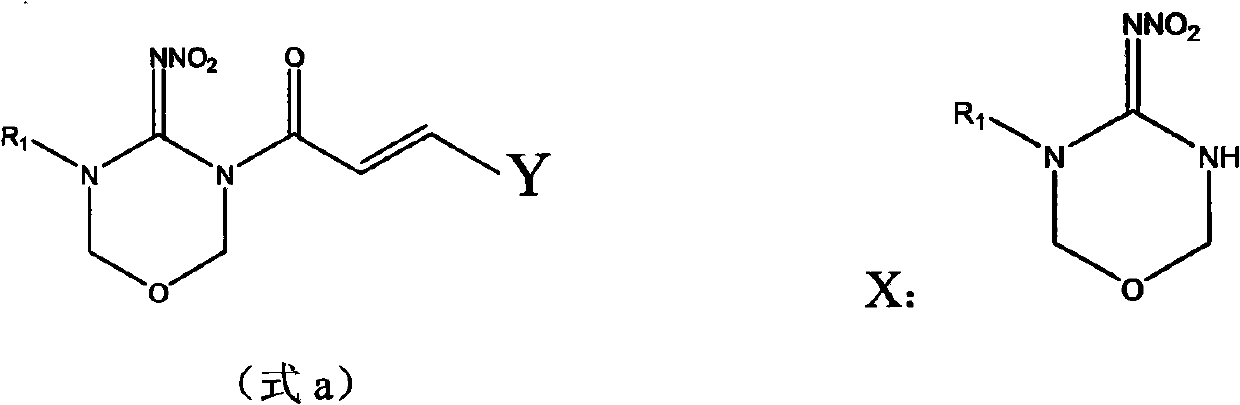
[0029] Example 1, (2E)-3-(2,4-dichlorophenyl)-1-(5-isopropyl-4-nitroimino-1,3,5-oxadiazin-3-yl )-propenone (YSX-18) preparation.
[0030] In a 50mL three-necked flask, put 0.98g of 5-isopropyl-4-nitroimino-1,3,5-oxadiazine, 1.5g of piperidine and 15mL of acetonitrile, stir, and then add 1.20g of 2, The acetonitrile solution of 4-dichlorophenylacryloyl chloride was added dropwise and reacted at 40°C for 5h. The reaction solution was cooled to room temperature, and the solvent was removed under reduced pressure to obtain a red liquid. Then the product after removing the solvent is directly subjected to chromatographic separation, the eluent is a mixed solution of ethyl acetate and petroleum ether (V:V=1:1), and the solvent is precipitated under reduced pressure to obtain 0.75 g of a white solid, which is (2E)-3-(2,4-Dichlorophenyl)-1-(5-isopropyl-4-nitroimino-1,3,5-oxadiazin-3-yl)-propenone (YSX-18), yield 60.7%. That 1 H NMR spectrum data are shown in Table 3.
[0031] Ot...
Embodiment 2
[0050] Embodiment 2, the biological activity of compound of the present invention to aphid
[0051] Positive control agent (E)-1,5-diphenyl-2-ene-1-pentanone (I) and the obtained compound sample of Example 1 were weighed 12mg ( 100%) compound sample, then take 2ml of acetone / methanol (1:1) mixed solvent with a 1-5ml pipette gun and add it to the weighing bottle. After it is fully dissolved, add 18ml of an aqueous solution containing 0.1% Tween-80, fully Mix well to obtain a 600 μg / mL assay solution. Select hibiscus leaves with aphids, leave 3-day-old nymphs, immerse the leaves with aphids in the liquid medicine for 5 seconds, record the number of insects after drying, put them into a petri dish with moisturizing filter paper, cover them and put them in (25± 1) In the light incubator at ℃. Each agent treats more than 30 heads. Check the results after 24 hours. The criteria for judging the death are: touch the insect body lightly, and the individual pests that cannot crawl n...
Embodiment 3
[0054] Embodiment 3, the biological activity of compound of the present invention to Plutella xylostella
[0055] Positive control agent (E)-1,5-diphenyl-2-ene-1-pentanone (I) and the compound sample obtained in Example 1 are respectively weighed 12mg in a 20ml weighing bottle with a ten-thousandth balance (100%) compound samples, then use a 1-5mL pipette gun to take 2mL of acetone / methanol (1:1) mixed solvent into the weighing bottle, after it is fully dissolved, add 18ml of aqueous solution containing 0.1% Tween-80, Mix well to obtain a 600 μg / mL assay solution. Spinosyn 10 μg / mL was prepared in the same way. Use a hole puncher to punch clean cabbage leaves into discs with a diameter of 2 cm, soak them in the liquid medicine for 5 seconds, put the leaves back up into a petri dish with a diameter of 6 cm with moisturizing filter paper, and insert 10 instar larvae of Plutella xylostella xylostella. Heads were covered and placed in a light incubator at (27±1)°C. Check the re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com