Catalyst for preparing propylene carbonate and application thereof
A technology of propylene carbonate and catalyst, applied in the field of catalyst for preparing propylene carbonate, can solve the problems of high catalyst preparation cost, unfavorable industrial production application and the like, and achieve the effects of less catalyst dosage, easy separation and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
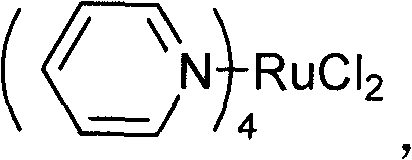
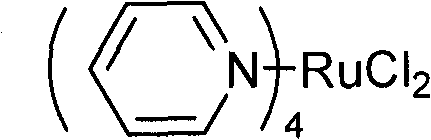
[0024] 0.12×10 -3 Moles of main catalyst Ru(py) 4 Cl 2 And 0.12×10 -3 Moles of co-catalyst cetyltrimethylammonium bromide was put into a pre-dried 100mL stainless steel autoclave, vacuum dried, the air in the kettle was replaced with a high-purity nitrogen device, and 16mL of purified propylene oxide (0.228 Mol), heating to 80°C, introducing carbon dioxide to 3MPa, reacting for 4 hours, and cooling to stop the reaction. The reaction liquid was transferred to a vacuum distillation device, and vacuum distillation was performed to collect the 76-78°C / 3mmHg fraction, which is propylene carbonate, weighing 23.0 grams, and the yield was 98.6%. The distillation residue is transferred to the autoclave to be used as a catalyst for the next catalytic reaction.
Embodiment 2
[0026] The residue from the distillation of propylene carbonate in Example 1 was transferred to the same autoclave as in Example 1, and recycled as a catalyst. 16ml of purified propylene oxide was added again to perform the reaction. The reaction conditions were the same as in Example 1. 22.9 g of propylene carbonate was obtained, and the yield was 98.3%.
Embodiment 3
[0028] Using the same equipment as in Example 1, under the same conditions, the co-catalyst replaced cetyltrimethylammonium bromide with tetrabutylammonium bromide, and the reaction was carried out. The temperature was raised to 100°C and carbon dioxide was introduced. To 2.5MPa, react for 6 hours to obtain 22.4 grams of propylene carbonate with a yield of 96.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com