Encoding sequence for Late Embryogenesis Abundant protein of Jatropha curcas and application in plants
A technology of jatropha curcas and leprosy, applied in the fields of molecular biology and genetic engineering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] Example 1 Cloning of the LEA protein gene of Jatropha curcas
[0094] 1. Organization separation (isolation)
[0095] The seeds of Jatropha curcas come from the Panzhihua area of Sichuan Province. The seeds of Jatropha curcas are collected and stored in the laboratory.
[0096] 2. RNA isolation (RNA isolation)
[0097] Peel off the seed coat of Jatropha curcas seeds, take out the seed kernels, grind them in a mortar, add liquid nitrogen, grind them into powder, take 100mg and transfer to a 1.5mL EP tube to extract total RNA (two-step lysis method) . Use formaldehyde denaturing gel electrophoresis to identify the quality of total RNA, and then measure the RNA content on a spectrophotometer.
[0098] 3. Cloning of Full-length cDNA
[0099] According to the amino acid conservative sequence of some plant LEAs, design merging primers, using the principle of homologous gene cloning, SMARTTM RACE cDNA amplification method (Clonetech kit) for full-length cDNA cloning in four stages:
...
Embodiment 2
[0109] Example 2 Sequence information and homology analysis of Jatropha curcas LEA protein gene
[0110] The length of the full-length cDNA of the new Jatropha curcas LEA protein is 1070 bp, and the detailed sequence is shown in SEQ ID NO. 1, in which the open reading frame is located at the 59-820 nucleotides. According to the full-length cDNA, the amino acid sequence of the LEA protein of Jatropha curcas was deduced, with a total of 254 amino acid residues, a molecular weight of 26454.62, and a pI of 4.64. See SEQ ID NO.2. for the detailed sequence.
[0111] The full-length cDNA sequence of the Jatropha curcas LEA protein and its encoded protein were used BLAST program to perform nucleotide summation in Non-redundant GenBank+EMBL+DDBJ+PDB and Non-redundant GenBank CDStranslations+PDB+SwissProt+Superdate+PIR databases The protein homology search revealed that it has 67% homology with the Arabidopsis LEA protein (NM_113148) at the nucleotide level (see Table 2); at the amino acid ...
Embodiment 3
[0112] Example 3 Prediction of the structure and type of Jatropha curcas LEA protein
[0113] 1. The structural domain analysis of Jatropha curcas LEA protein
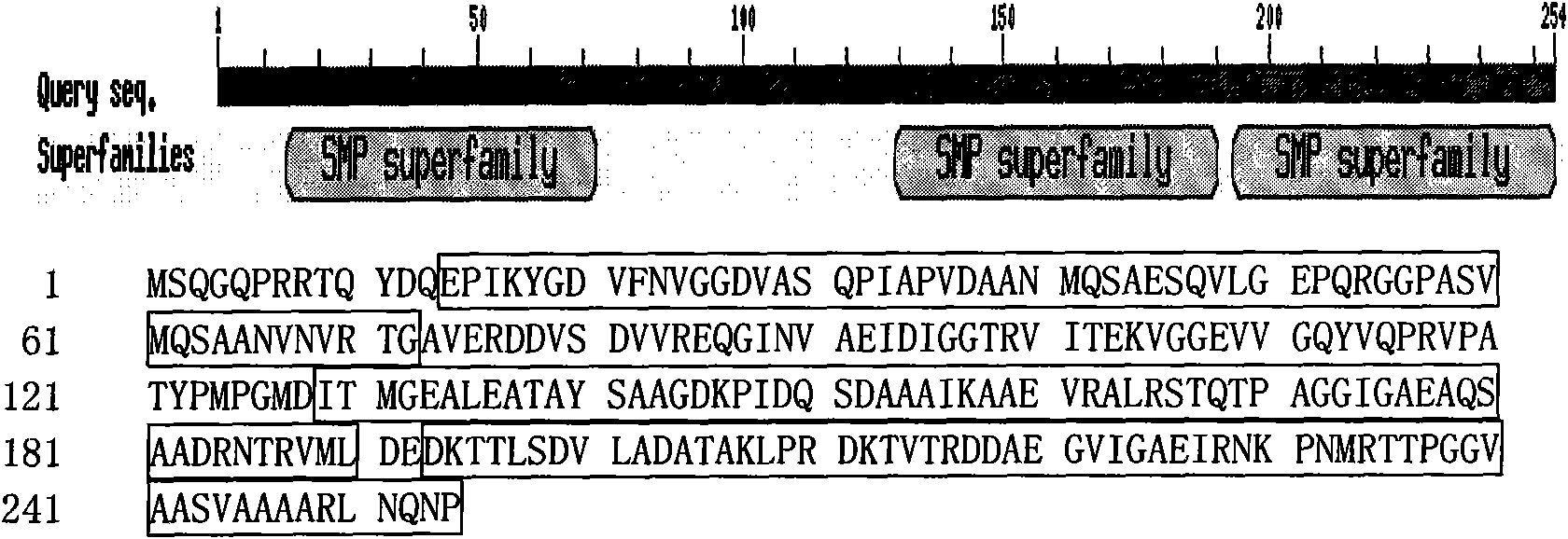
[0114] The amino acid sequence of the LEA protein of Jatropha curcas was searched for the domain in the NCBI database (website: http: / / www.ncbi.nlm.nih.gov / Blast.cgi), and the results are as attached figure 1 Shown.
[0115] (1) In the amino acid sequence, there are the following domain regions: the framed region (ie SEQ ID. 1 in the sequence listing is from the amino-terminal (N-terminal) amino acid residues 14-72, 129-190, 193-254 Amino acid residues). The framed area constitutes the SMP mature protein functional module (Seed maturation protein, SMP).
[0116] (2) Functional analysis There is a functional module of seed mature protein in the amino acid sequence of the gene, so it can be predicted that it does have corresponding functions.
[0117] 2. Classification prediction of Jatropha curcas LEA protein
[0118] The amino...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com