Pure perovskite phase rare earth ferrite porous hollow sphere, preparation method and application thereof
A ferrite and hollow sphere technology, applied in separation methods, chemical instruments and methods, dispersed particle separation, etc., can solve the problems of limited precious metal catalyst resources, poor reusability, and easy agglomeration and growth of precious metal nanoparticles. The preparation method is green, the NO conversion rate is high, and the N2 selectivity is good.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
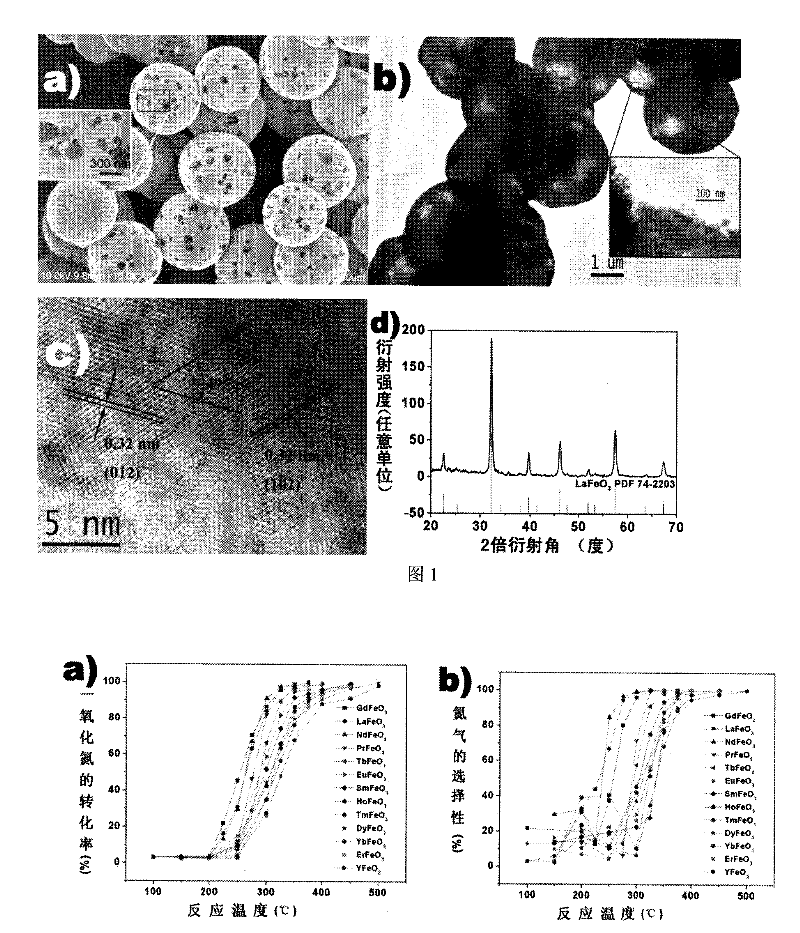
[0033] (2) Preparation of porous hollow spheres of rare earth ferrite: put the rare earth / iron composite citric acid complex precursor in a tube furnace and calcinate at 700-900°C for 1-3 hours, and cool to room temperature to obtain rare earth ferrite Salt porous hollow sphere.
[0034] The product is used as a catalyst to catalyze the reduction of CO to NO to generate N 2 The reaction steps are as follows: Weigh 50 mg of catalyst and fill it into a vertical quartz tube column reactor, and place it in the middle of the vertical tube furnace. The catalyst was pretreated by heating to 100 °C for 1 hour under the protection of nitrogen atmosphere and then cooled to room temperature. Mix NO, CO and He at a volume ratio of 5:10:85 and pass them into a vertical quartz column reactor, keeping the space velocity at 24000h -1 . The quartz column reactor starts to heat, and the reaction product gas at different temperatures is extracted by 1cm 3 Pass through a gas chromatograph for...
Embodiment 1
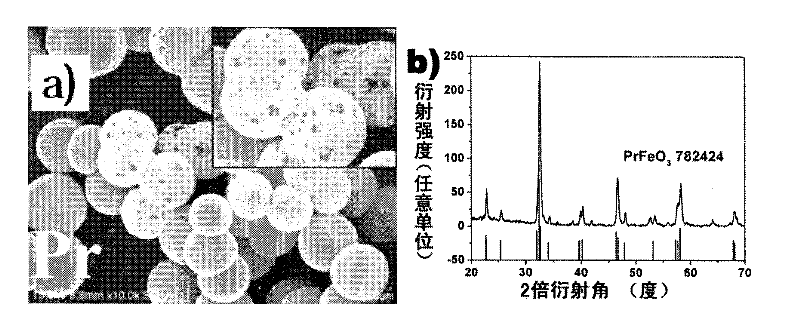
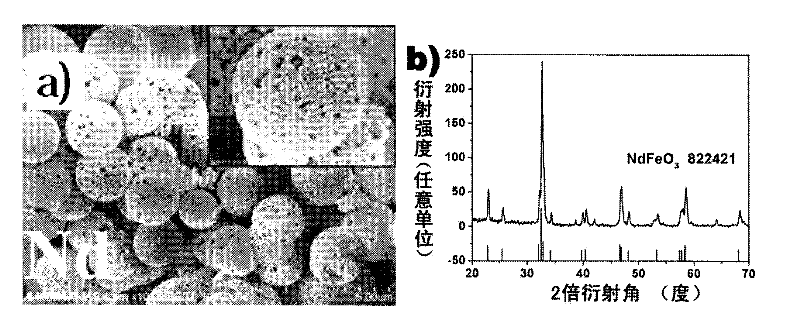
[0036] In a 50 ml beaker, add 271 mg of La(NO 3 ) 3 ·6H 2 O and 252 mg of Fe(NO 3 ) 3 9H 2 O, add 25 milliliters of deionized water again, stir at room temperature for 30 minutes to make it dissolve completely. Then add 788 mg of citric acid monohydrate to the beaker, and continue to stir at room temperature for 30 minutes, then transfer the mixed solution to a 30 ml autoclave. After sealing, put the autoclave into an oven and react at 180°C for 24 hours. After naturally cooling to room temperature, the obtained product was washed three times with deionized water and absolute ethanol, respectively, and dried at 80° C. for 3 hours. The dried product was calcined in a tube furnace at 800°C for 2 hours, and cooled to room temperature to obtain porous hollow lanthanum ferrite spheres. Figure 1a is a scanning electron microscope photo of the product. It can be seen that the product is a spherical structure, the outer diameter of the ball is between 2 and 5 microns, and the s...
Embodiment 2
[0038] In a 50 ml beaker, add 217 mg of La(NO 3 ) 3 ·6H 2 O and 202 mg of Fe(NO 3 ) 3 9H 2 O, add 25 milliliters of deionized water again, stir at room temperature for 20 minutes to make it dissolve completely. Then add 630 mg of citric acid monohydrate into the beaker, and continue to stir at room temperature for 20 minutes, then transfer the mixed solution to a 30 ml autoclave. After sealing, put the autoclave into an oven and react at 180°C for 24 hours. After naturally cooling to room temperature, the obtained product was washed three times with deionized water and absolute ethanol, respectively, and dried at 80° C. for 3 hours. The dried product was calcined in a tube furnace at 800°C for 2 hours, and cooled to room temperature to obtain porous hollow lanthanum ferrite spheres.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com