LC-MS/MS analysis method for detecting morphinane alkaloid in whole blood and urine
An LC-MS and analytical method technology, applied in analytical materials, measuring devices, material separation, etc., can solve the problems of inability to meet the needs of case inspection, large polarity, difficulty in extraction and detection, and save analysis time and simplify The effect of the experimental procedure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
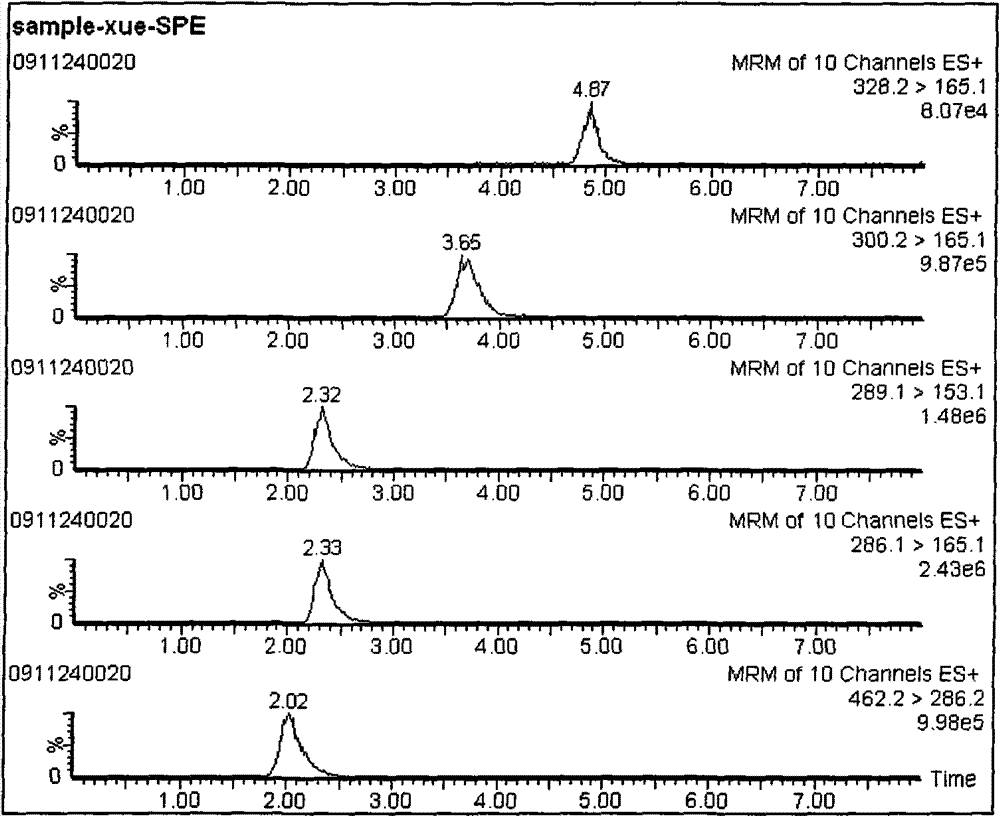
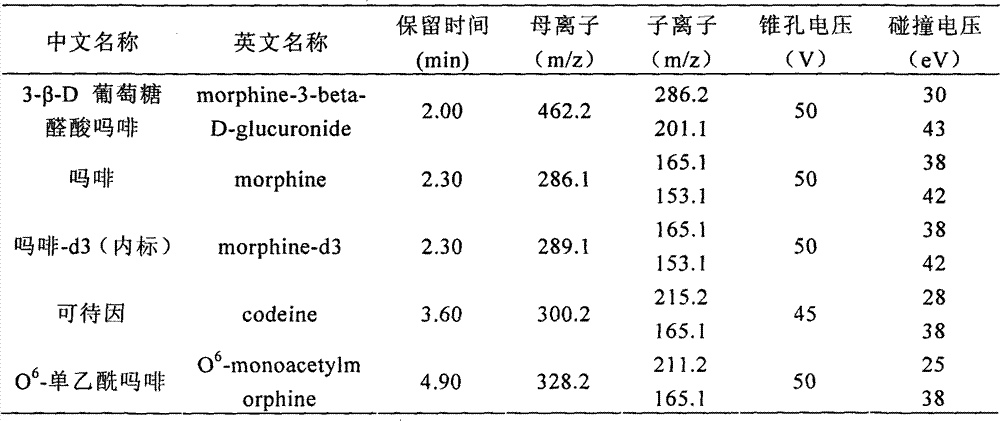
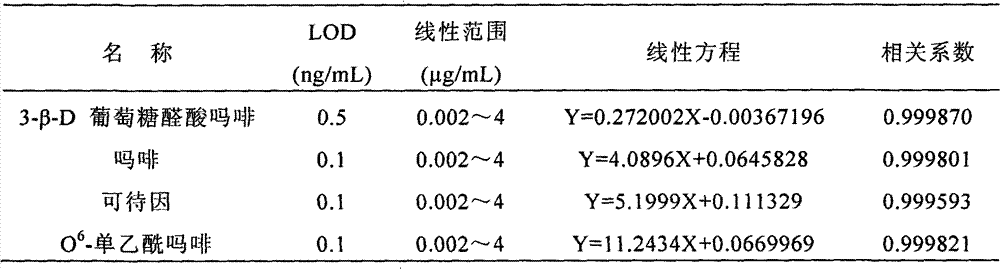
[0043] 3-β-D glucuronate morphine, morphine, O 6 - Detection of monoacetylmorphine, codeine
[0044] 1. Liquid chromatography conditions
[0045] Column: Xterra MSC 18 3.5μm 2.1×150mm (Waters Company)
[0046] Mobile phase is A and B, and wherein A is the water containing 2mmol / L ammonium formate and 0.05% (v / v) formic acid, B is the acetonitrile containing 2mmol / L ammonium formate and 0.05% (v / v) formic acid, A, The volume ratio of B is 90:10.
[0047] Column temperature: 45°C
[0048] Flow rate: 0.2mL / min
[0049] Injection volume: 10μL
[0050] 2. Mass Spectrometry Conditions
[0051] Detection method: multiple reaction monitoring (MRM)
[0052] Scanning method: Positive ion scanning
[0053] Electrospray voltage: 3200V
[0054] Nebulizing gas flow rate: N 2 600L / hr
[0055] Taper hole blowback flow rate: N 2 50L / hr
[0056] Ion source temperature: 105°C
[0057] Collision gas: argon
[0058] Internal standard: Morphine-d3
[0059] 3. MRM parameters
...
Embodiment 2
[0077] 3-β-D glucuronomorphine, morphine, O 6 - Detection of monoacetylmorphine, codeine
[0078] 1. Liquid chromatography conditions, mass spectrometry conditions, and MRM parameters are the same as in Example 1.
[0079] 2. Sample Processing
[0080] Activate the oasis HLB column (1cc / 30mg) with 1mL methanol and 1mL water in sequence, accurately draw 1.0 urine sample, add internal standard 100ng, mix well and put on the column, rinse the column with 1mL water containing 5% (v / v) methanol , and then eluted with 1mL of methanol, the flow rate of the whole process was controlled at about 2mL / min. The eluate was blown dry in a water bath at 60°C under air flow, and the residue was dissolved in 100 μL of the initial mobile phase, and transferred to an autosampler vial for injection.
[0081] 3. Result analysis
[0082] 1) Chromatographic behavior
[0083] 3-β-D Glucuronate Morphine, Morphine, O 6 - The retention times of monoacetylmorphine, codeine and internal standard und...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com