Preparation method of submicron level LiniO.5MnO.5O2 cathode material
A cathode material, sub-micron technology, applied in battery electrodes, nickel oxide/nickel hydroxide, electrical components, etc., to achieve the effects of low production cost, easy process control, and excellent cycle stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
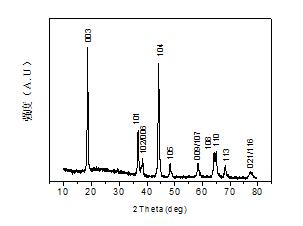
Image
Examples
Embodiment 1
[0020] Accurately weigh 2.8088g nickel sulfate heptahydrate (0.01mol) and 1.6901g manganese sulfate monohydrate (0.01mol), and dissolve them in a 100mL beaker successively to prepare 0.01mol / L Ni 2+ and Mn 2+ mixture. Prepare 100mL0.04mol / L NaOH solution, then add ammonia water at a molar ratio of 1:0.2 relative to NaOH, and the mixed solution of NaOH and ammonia water is used as the precipitant solution. Use a titration device to slowly drop the above metal ion mixed solution and precipitant solution into 100mL aqueous solution in which 14mg (0.14g / L) sodium lauryl sulfate is dissolved at a speed of 1.0mL / min and 1.2mL / min respectively . Before titration, sodium lauryl sulfate had been completely dissolved in the aqueous solution and kept stirring for 1 h, the stirring speed was 300 r / min, and the reaction temperature was 55°C. After the titration is complete, continue stirring at high speed for 5 h. After the precipitation was complete, the turbid solution was left to st...
Embodiment 2
[0025] Accurately weigh 2.8088g nickel sulfate heptahydrate (0.01mol) and 1.6901g manganese sulfate monohydrate (0.01mol), and dissolve them in a 100mL beaker successively to prepare 0.01mol / L Ni 2+ and Mn 2+ mixture. Prepare 100ml of 0.04mol / L NaOH solution, then add ammonia water at a molar ratio of 1:0.2 relative to NaOH, and a mixed solution of NaOH and ammonia water as a precipitant solution. Use a titration device to slowly drop the above metal ion mixed solution and precipitant solution into 100mL of 40mg (0.40g / L) sodium dodecylbenzenesulfonate solution at a speed of 1.0mL / min and 1.2mL / min respectively. in aqueous solution. Other steps are the same as in Example 1.
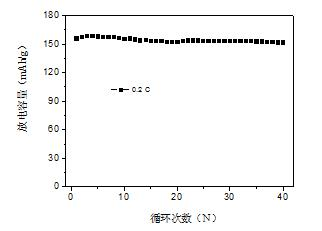
[0026] Figure 5 (a) The capacity retention diagram of the half-cell with the prepared material as the positive electrode in the first 20 cycles is recorded. It can be seen from the figure that the first discharge specific capacity of the prepared material at 0.2C rate is 158.3mAh / g, 20 cycles The po...
Embodiment 3
[0028] Accurately weigh 2.4884g of nickel acetate tetrahydrate (0.01mol) and 2.6810g of manganese acetate dihydrate (0.01mol), and dissolve them in a 100mL beaker to prepare 0.01mol / L Ni 2+ and Mn 2+ mixture. Prepare 100ml of 0.04mol / L NaOH solution, then add ammonia water at a molar ratio of 1:0.2 relative to NaOH, and a mixed solution of NaOH and ammonia water as a precipitant solution. Use a titration device to slowly drop the above-mentioned metal ion mixed solution and precipitant solution into 100 mL of an aqueous solution in which 40 mg (0.40 g / L) of polyethylene glycol 2000 is dissolved at a speed of 1.0 mL / min and 1.2 mL / min, respectively. Other steps are the same as in Example 1.
[0029] Figure 5 (b) Recorded the capacity retention diagram of the half-battery with the prepared material as the positive electrode in the first 20 cycles. It can be seen from the figure that the first discharge specific capacity of the prepared material at 0.2C rate is 155.4mAh / g, 20...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com