The preparation method of felodipine slow-release tablet
A kind of gentle and lodipine technology, which is applied in the field of preparation of felodipine sustained-release tablets, can solve the problems of difficult industrialized production, cumbersome process, and high production cost, and achieves the advantages of easy preparation method, high product yield and reduced production cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
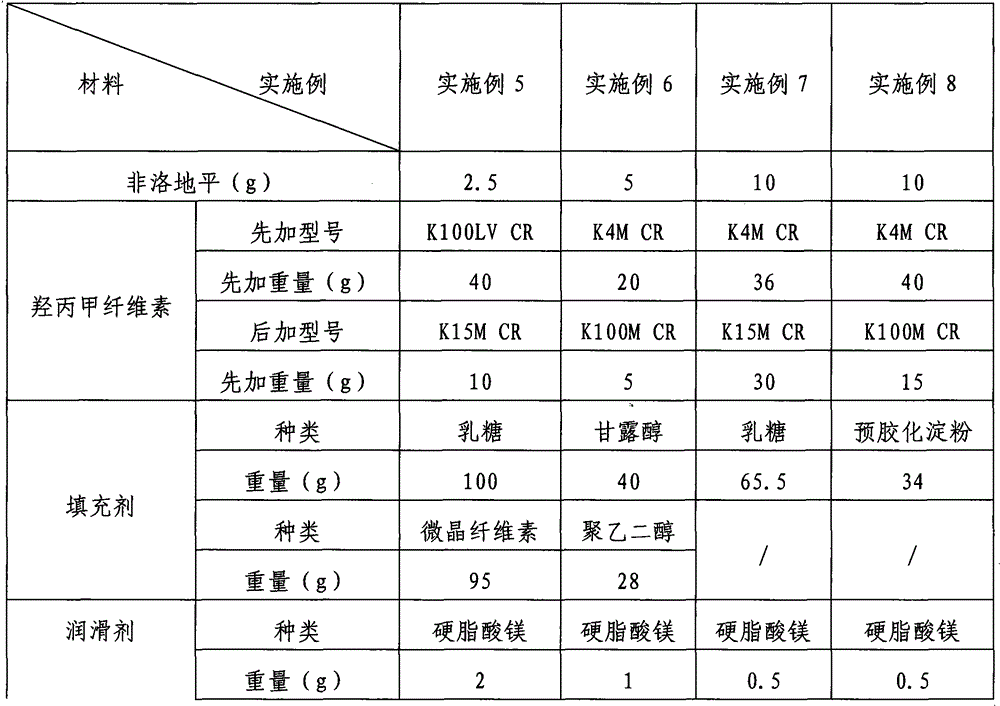
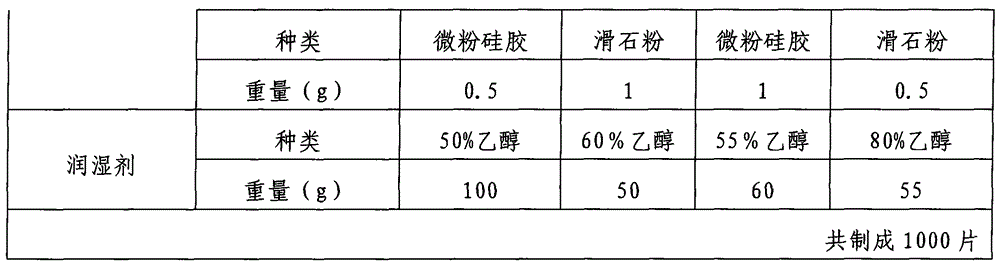
Examples
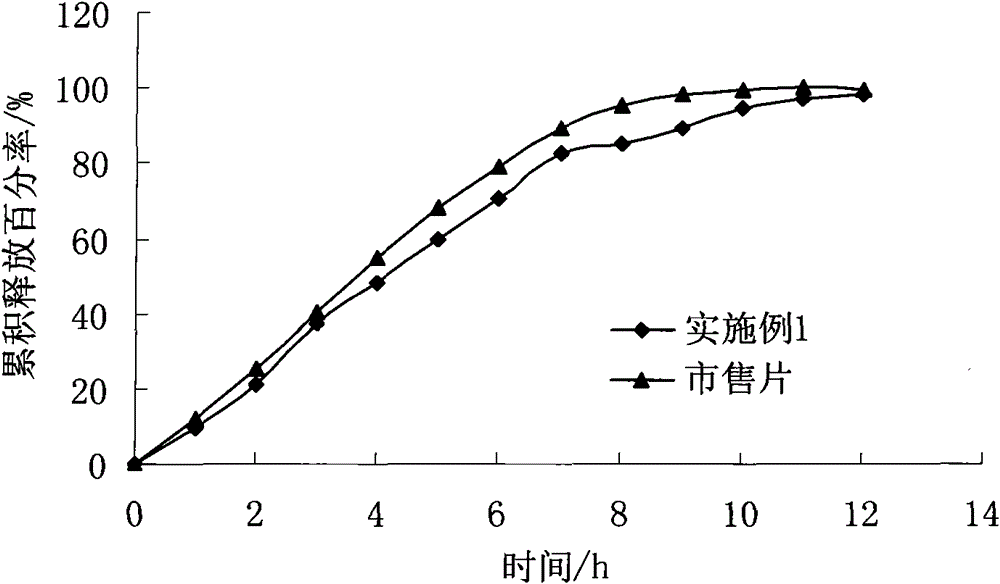
Embodiment 1
[0028] Preparation of Felodipine Sustained-release Tablets
[0029] prescription:
[0030] Felodipine 5g
[0031] Hypromellose K100LV CR 50g
[0032] Hypromellose K4M CR 28g
[0033] Lactose 115g
[0035] 65% ethanol appropriate amount
[0036] A total of 1000 pieces were made
[0037] Preparation Process:
[0038] 1. Preparation of tablet cores: Take felodipine, hypromellose K100LV CR and lactose in the prescribed amount, mix them evenly, add about 80 g of 65% ethanol solution to make soft material, sieve to make wet granules, 50-60 ℃ drying to a water content of 2-3%, granulation, adding the prescribed amount of hypromellose K4M CR, magnesium stearate, tableting, to obtain tablet cores. The content of felodipine is 2.5%.
[0039] 2. Coating: pan-coat with Opadry, increase the weight by 2 to 3%, and make slow-release tablets containing 5 mg of felodipine.
Embodiment 2
[0041] Felodipine 5g
[0042] Hypromellose K100LV CR 29.4g
[0043] Hypromellose K4MCR 10.3g
[0044] Hypromellose K15M CR 11.8g
[0045] Mannitol 59.7g
[0046] Macrogol 6000 29.4g
[0047] Magnesium stearate 0.7g
[0049] 65% ethanol appropriate amount
[0050] A total of 1000 pieces were made
[0051] Preparation Process:
[0052] 1. Preparation of tablet cores: take by weighing felodipine, hypromellose K100LV CR, mannitol, and polyethylene glycol 6000 of the prescribed amount, mix evenly, add about 65g of 65% ethanol solution to make soft material, sieve, Make wet granules, dry at 50-60°C until the L.O.D is 2-3%, granulate, add the prescribed amount of hypromellose K4M CR, hypromellose K15M CR, magnesium stearate and talc, and press into tablets , controlling the tablet hardness to 4-7kg to obtain tablet cores. The main drug content is 3.3%.
[0053] 2. Coating: use Opadry, pan-coat until the tablet core increases in wei...
Embodiment 3
[0055] Felodipine 2.5g
[0056] Hypromellose K4M CR 15g
[0057] Hypromellose K15M CR 5g
[0058] Lactose 16g
[0059] Pregelatinized starch 33g
[0060] Microcrystalline Cellulose 27.5g
[0061] Magnesium stearate 0.5g
[0062] Micronized silica gel 0.5g
[0063] 70% ethanol appropriate amount
[0064] A total of 1000 pieces were made
[0065] Preparation Process:
[0066] 1. Preparation of tablet cores:
[0067] Weigh the prescribed amount of felodipine, hypromellose K4M CR, lactose, pregelatinized starch and microcrystalline cellulose, mix well, add about 50g of 70% ethanol solution to make soft material, and sieve to make wet granules , dried at 50-60°C until the L.O.D is 2-3%, sieved and granulated, added hypromellose K15M CR, magnesium stearate and micro-powdered silica gel in the prescribed amount, and pressed into tablets to control the hardness of the tablets to 3-5kg. Obtain the core. The main drug content is 2.5%.
[0068] 2. Coating: use O...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com