Method for preparing and purifying cuprous halide
A cuprous halide and purification method technology, applied in the direction of copper halide, copper chloride, etc., can solve the problems of complex equipment and process, small product particles, pollution of the environment, etc., and achieve the effect of economical reaction process, high purity and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment one: the preparation of cuprous chloride
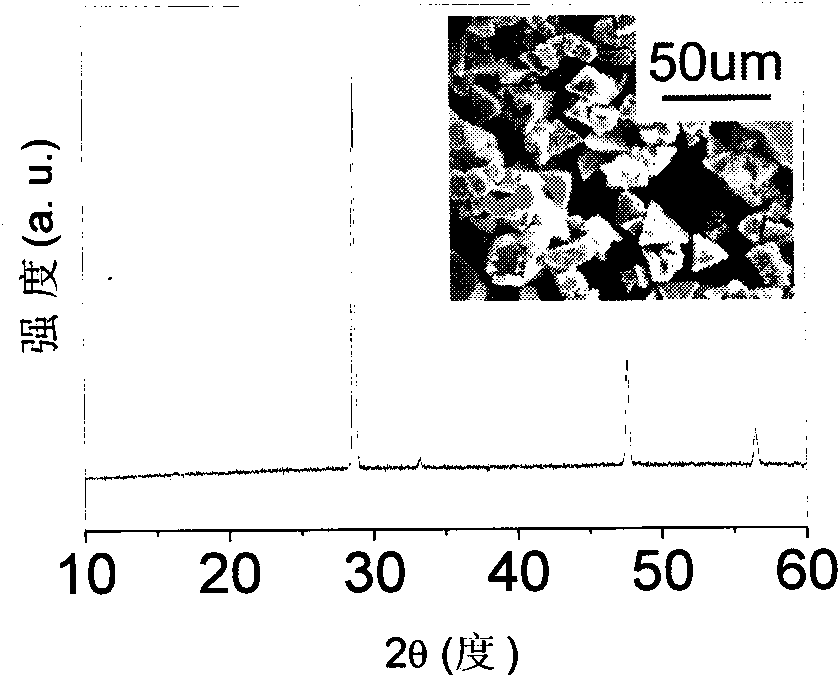
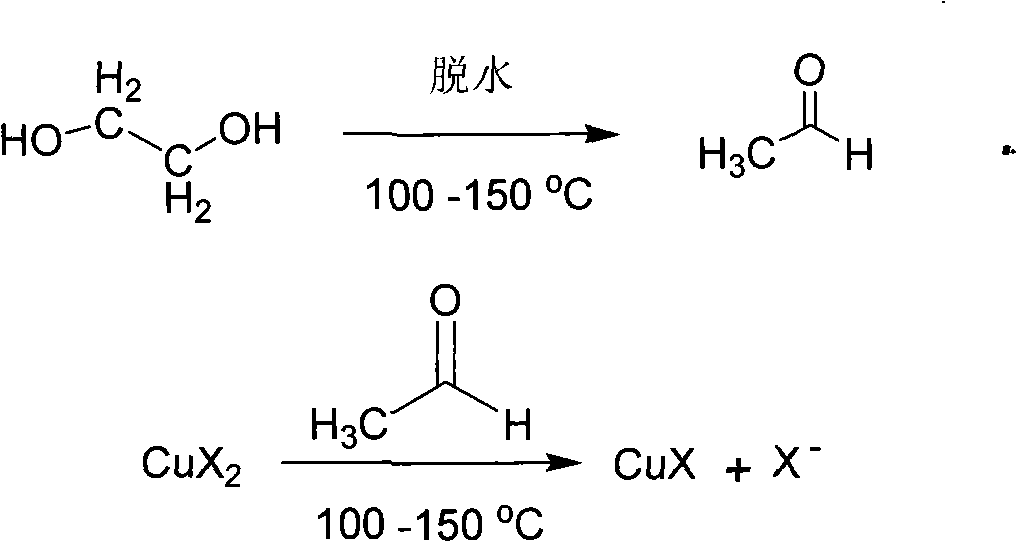
[0023] Weigh 2.50g copper chloride hydrate (CuCl 2 2H 2 O) put into 50mL glass test tube, then add 40mL of ethylene glycol solvent, dissolve copper chloride hydrate (CuCl 2 2H 2 O) A grass-green solution is obtained. Close the mouth of the test tube with a rubber stopper with an open hole (diameter of the small hole is about 1 mm). Heat and maintain the temperature of the ethylene glycol solvent at around 160°C. After one day, the color of the solution becomes light, and there is a white precipitate at the bottom of the test tube. The white precipitate was washed twice with deionized water, and then dried in a vacuum oven at 60° C. for 8 hours.
[0024] Adopt X-ray powder diffraction technique to carry out phase analysis to product, the result is as follows figure 2 shown. The positions of all diffraction peaks are consistent with the standard spectrum, indicating that the obtained product is cuprous chloride...
Embodiment 2
[0025] Embodiment two: the purification of cuprous bromide
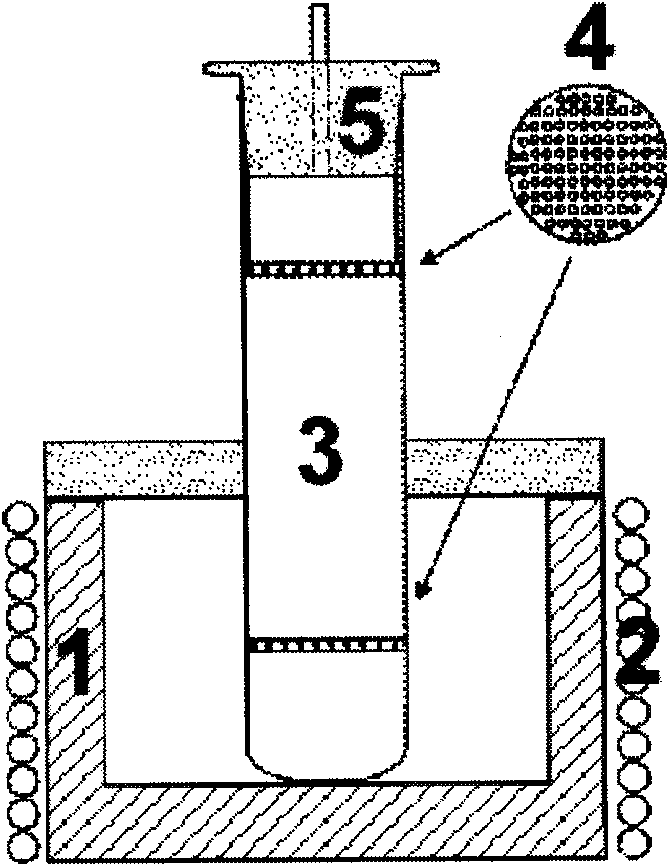
[0026] Put 4.0g of cuprous bromide (purity less than 95%) and 2.0g of potassium bromide (analytical pure) into the bottom of a glass container with a capacity of 100mL, and place a partition at the bottom and upper 1 / 5 of the container . Pour 90ml of ethylene glycol solvent (analytical grade), and seal the mouth of the glass container with a perforated rubber stopper. Heating the bottom of the glass container, keeping the temperature at the bottom at around 170°C, and the temperature near the upper partition at around 150°C, after 12 hours, a large amount of white cuprous bromide polycrystalline particles were deposited on the surface of the upper partition. The cuprous bromide was taken out, washed twice with deionized water, and then dried in a vacuum oven at 60° C. for 8 hours.
[0027] The phase analysis of the product was carried out by X-ray powder diffraction technique, and the result showed that the obtaine...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com