High temperature resistant L-arabinose isomerase and application thereof
An arabinose, high temperature-resistant technology, applied in the field of bioengineering, can solve the problems of low optimal reaction temperature, poor thermal stability, unfavorable industrial applications, etc., and achieve good activity and stability, good thermal stability and pH tolerance , the effect of wide application prospects and economic significance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: Extraction of Lactobacillus fermentum NXTag-1 genomic DNA.
[0039] According to the instruction manual provided by the manufacturer, the genomic DNA of Lactobacillus fermentum (Lactobacillus fermentum) NXTag-1CGMCC NO.2921 in the logarithmic growth phase was extracted with the Genomic DNA Purification Kit (Takara, Dalian), and 8 g / L agarose was used to coagulate The obtained bacterial genomes were detected by gel electrophoresis.
Embodiment 2
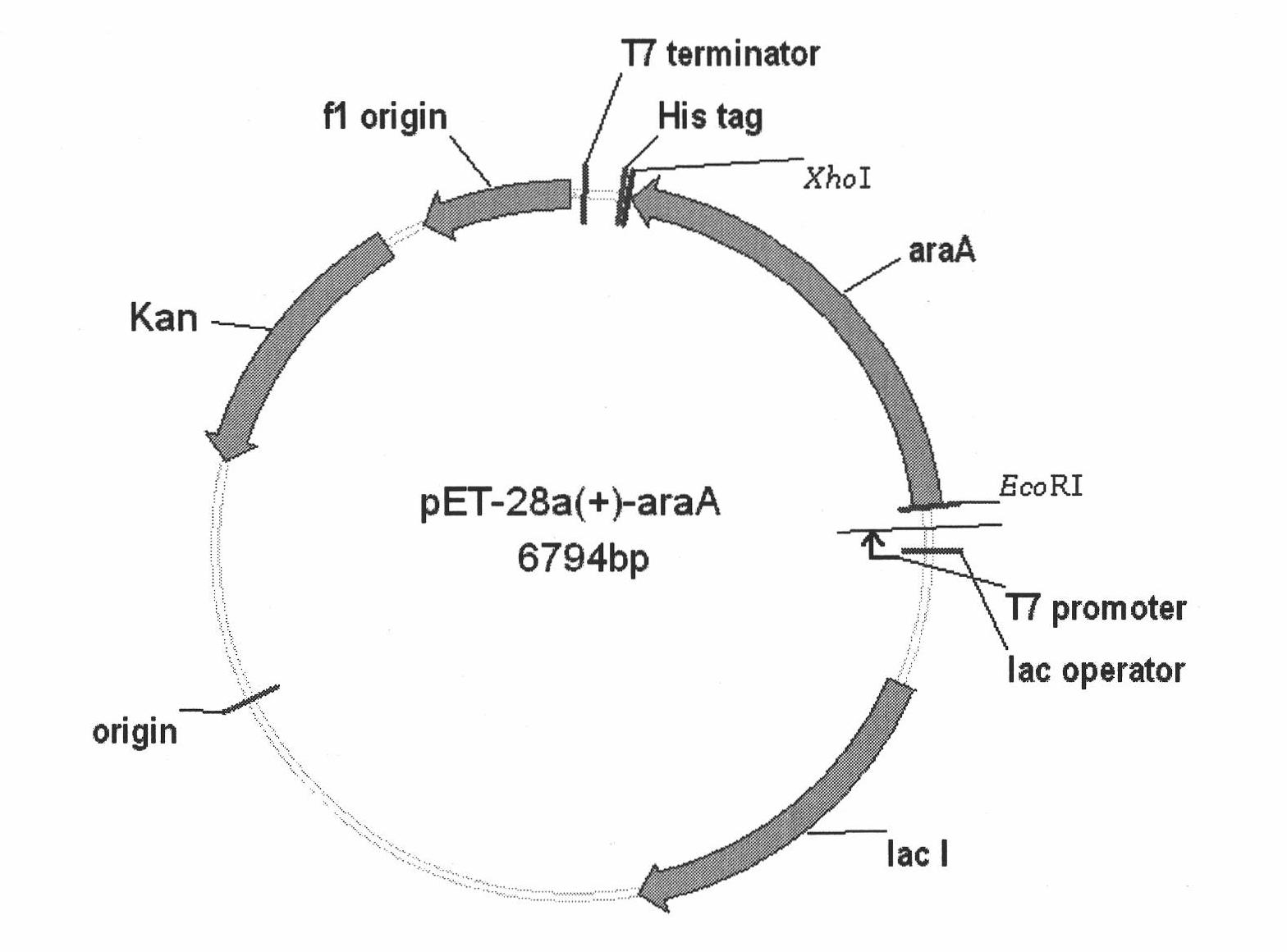
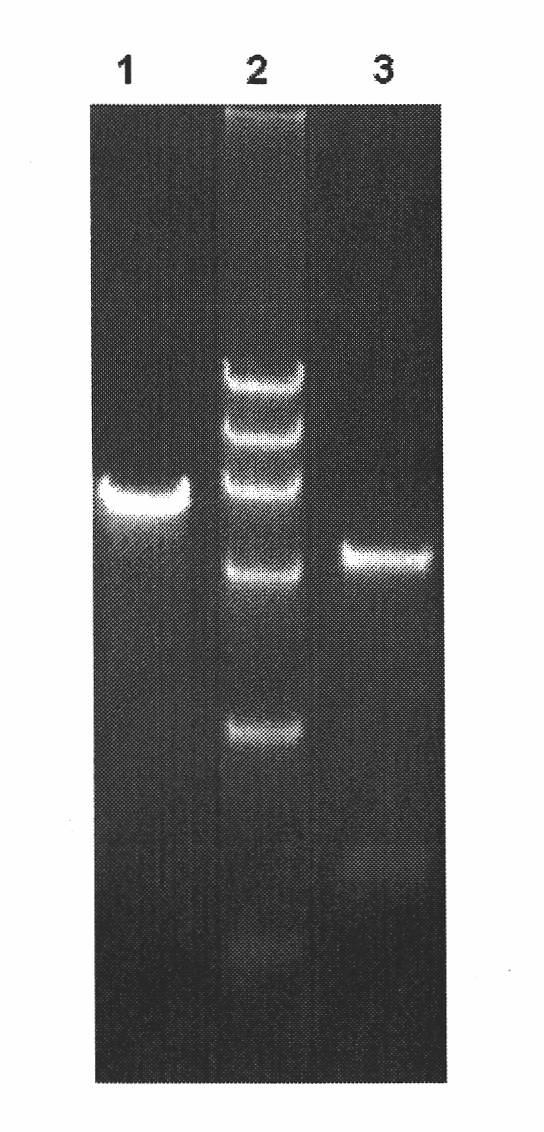
[0040] Example 2: Cloning of the gene encoding L-arabinose isomerase (araA) and construction of recombinant bacteria.
[0041] 2.1 PCR amplification of araA gene
[0042] According to the sequence of the L-AI gene of Lactobacillus fermentum reported on Gene Bank, use Vector NTI software to design primers Primer1 and Primer2, the primer sequence is:
[0043] Primer1: 5'-AGAGAATTCATGCGTAAGATGCAAGATTAC-3'
[0044] Primer2: 5'-AAGCTCGAGCTACTTGATGTTGATAAAGT-3'
[0045] Using the genomic DNA of Lactobacillus fermentum obtained in Example 1 as a template, the gene fragment of Lactobacillus fermentum was amplified.
[0046] The PCR amplification system is: 2 μL of genomic DNA, 1 μL of each of primers Primer1 and Primer2, 2 μL of dNTP, 2.5 μL of 10×Tag buffer, 0.5 μL of ExTag polymerase, ddH 2 O 14 μL.
[0047] The PCR reaction program was: pre-denaturation at 94°C for 2 min, denaturation at 94°C for 2 min; then annealing at 60°C for 30 s, extension at 72°C for 1 min, and 35 cycles...
Embodiment 3
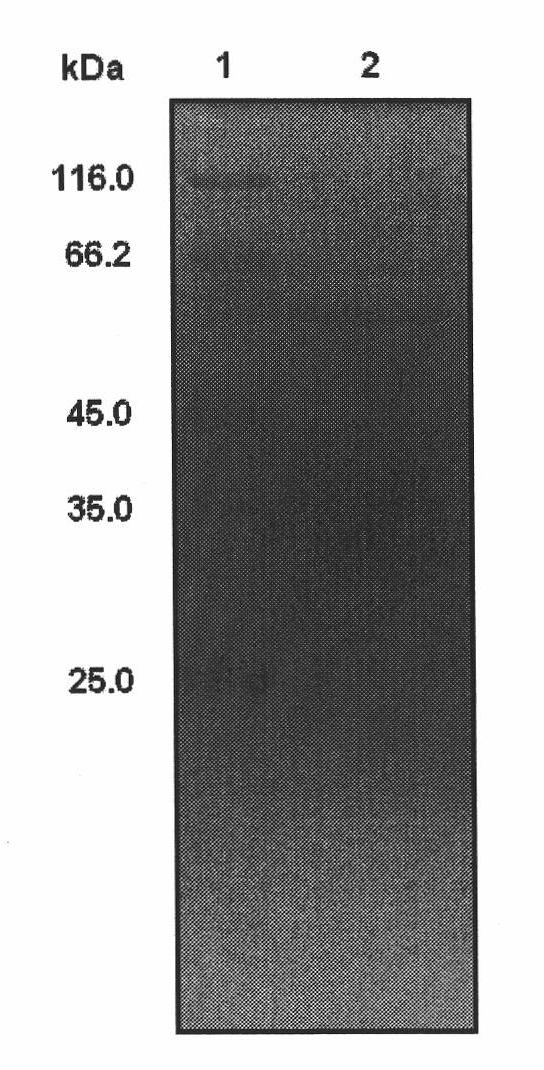
[0066] Example 3: Induced expression of L-arabinose isomerase.
[0067] Recombinant Escherichia coli BL21-AI was inoculated in 5 mL of LB liquid medium supplemented with 25 μg / mL kanamycin, and cultured on a shaker at 37°C overnight; then transferred to 100 mL of LB medium ( In a 500mL shake flask containing 25μg / mL kanamycin), culture on a shaker at 37°C for 2-3 hours, until OD 600 At about 0.6, add IPTG for induction (IPTG final concentration 1mmol / L), or add 1g / L lactose for induction, and then continue to induce expression for 6 hours, and collect the bacteria by centrifugation.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com