Freeze-dried powder injection of cefamandole nafate and preparation method thereof
A technology of cefamandole sodium and freeze-dried powder injection, which is applied in the field of preparation of freeze-dried powder injection, can solve the problems of blocked blood vessel embolism, achieve good stability, good solubility, and do not affect the effect of solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
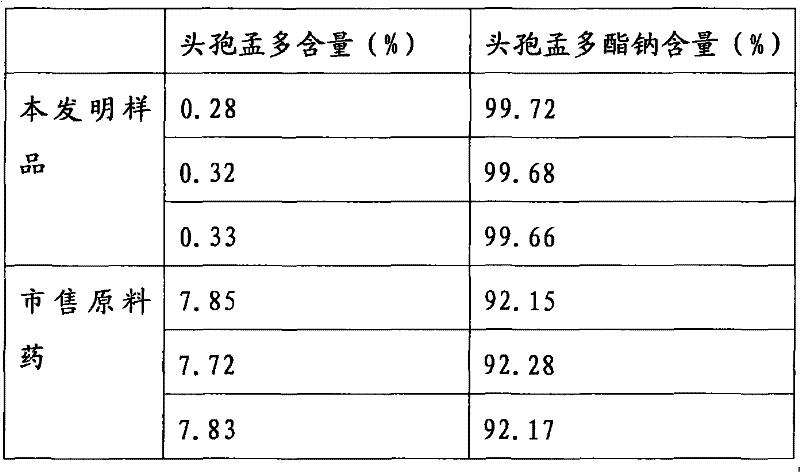
[0033] Take by weighing 200g of cefamandole sodium raw material, place in a 100ml beaker, add 50ml of water to dissolve. Filter with a 0.45 μm membrane, and freeze-dry the filtrate to obtain cefamandole sodium freeze-dried powder. Dissolve completely in water for injection within 25s.
Embodiment 2
[0035] Take by weighing 2000g of cefamandole sodium raw material, place in a 1000ml beaker, add 500ml of water to dissolve. Filter with a 0.22 μm membrane, and freeze-dry the filtrate to obtain cefamandole sodium freeze-dried powder. Dissolve completely in water for injection within 20s.
Embodiment 3
[0037] Take by weighing 2000g of cefamandole sodium raw material, place in a 1000ml beaker, add 500ml of water to dissolve. Filter with a 1.0 μm membrane, and freeze-dry the filtrate to obtain cefamandole sodium freeze-dried powder. Dissolve completely in water for injection within 30s.
[0038] Example 3
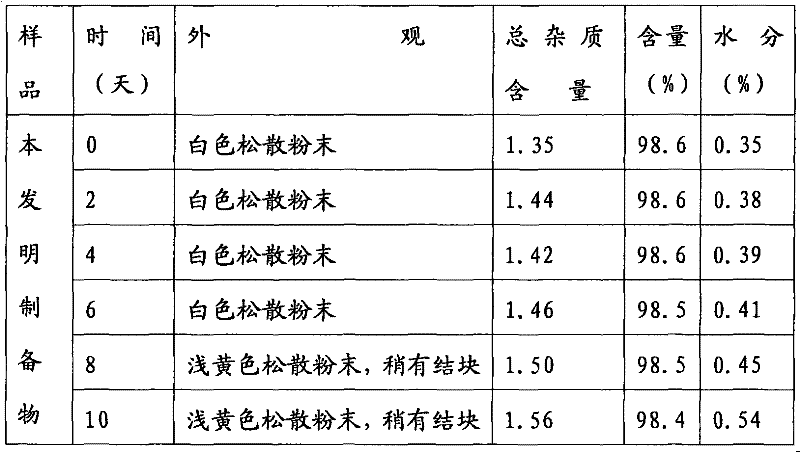
[0039] Take by weighing some cefamandole sodium freeze-dried powder injections, pass through a 60-mesh sieve after being pulverized, and carry out aseptic subpackaging according to the loading volume requirements under the condition of grade 100 in a sterile room to obtain cefamandole sodium for injection. Dry powder injection. The loading can be but not only 0.5g, 1g, 2g per bottle.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com