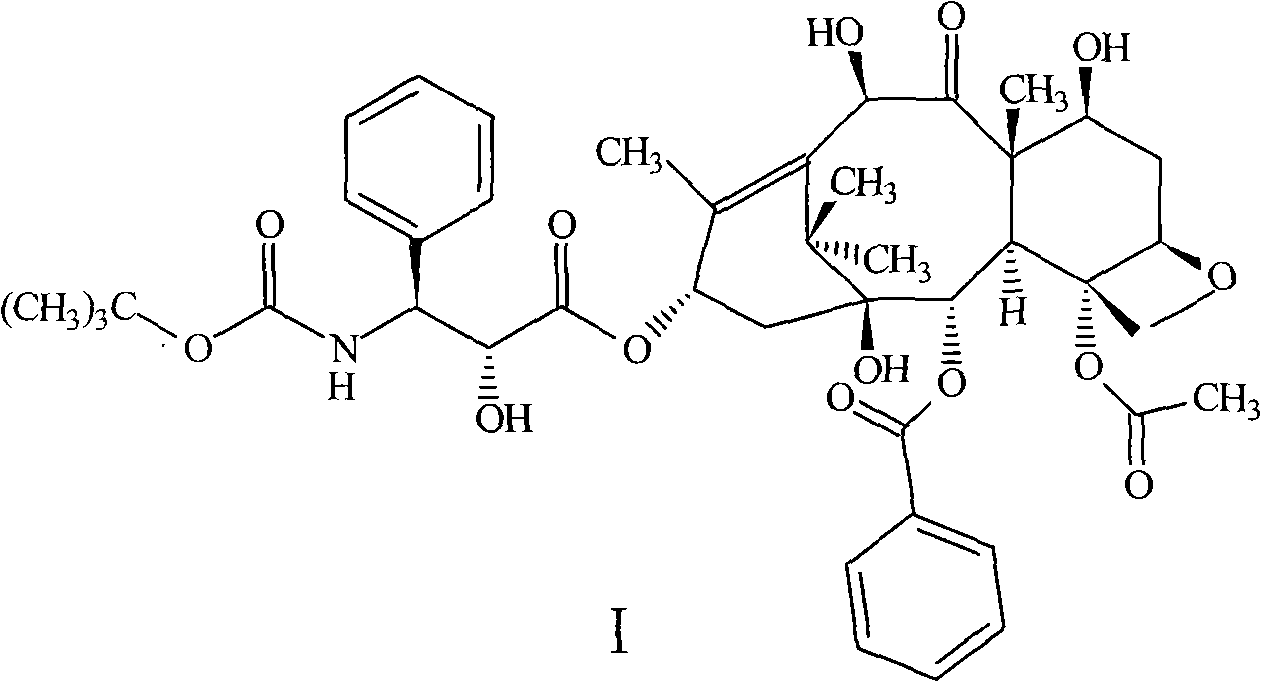
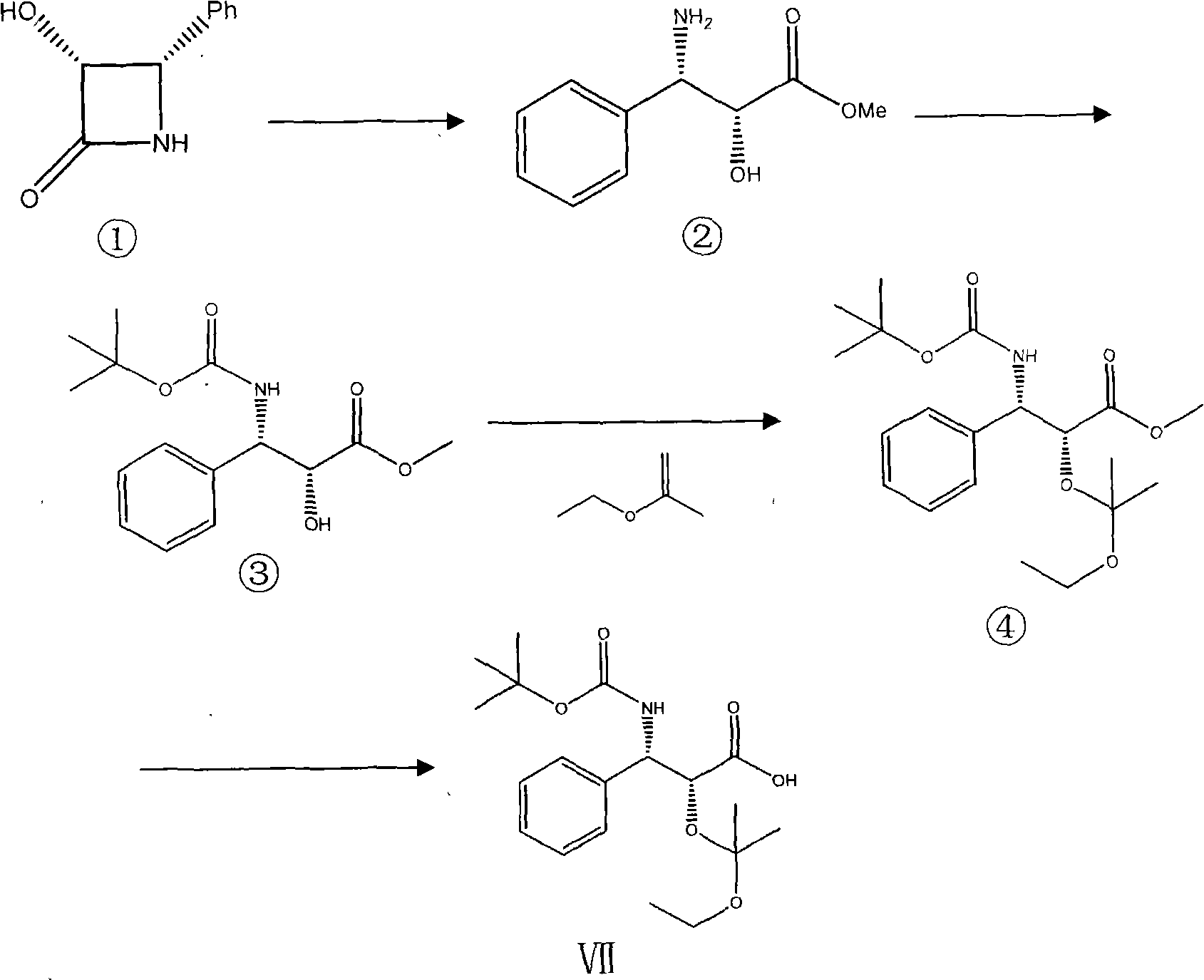
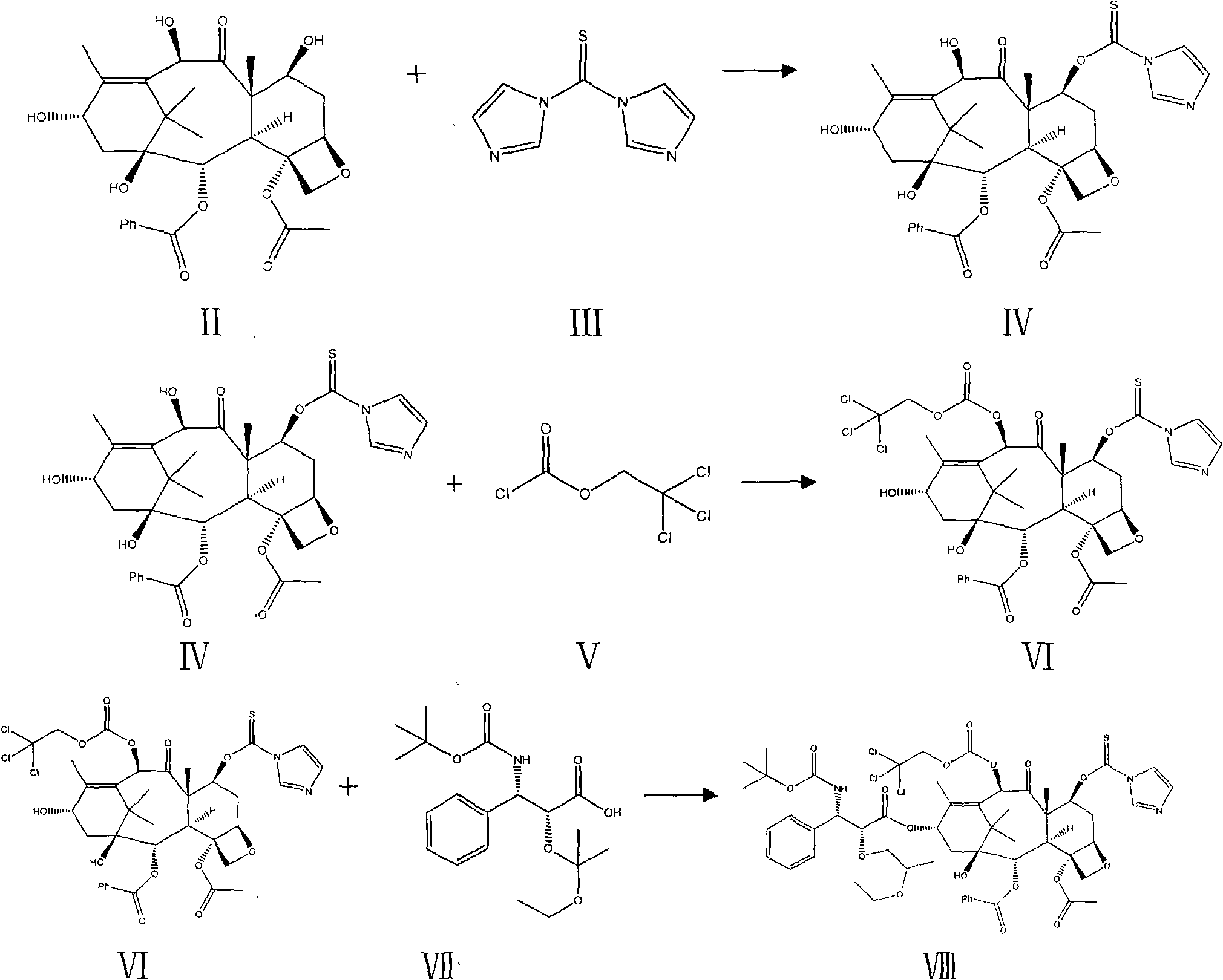
Compound and preparation method thereof and midbody compound prepared with same and preparation method thereof
A technology for compounds and intermediates, applied in the field of compounds and their preparation, can solve the problems of low synthesis yield, harsh conditions, unfavorable industrial operation, etc., and achieves soft reaction conditions, convenient industrial production, and superior chemical selectivity and yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Synthesis of Compound (Ⅶ)
[0023] Synthesis of compound ②: under nitrogen protection, 4.9 g (30 mmol) of compound ① was dissolved in 60 ml of methanol, 12 ml of 6N hydrochloric acid aqueous solution was added to the reaction solution, and reflux reaction was carried out at 65° C. for 20 hours, and the reaction solution was cooled to room temperature. In a water bath at 40°C, concentrate under reduced pressure to completely remove methanol, add 30ml of deionized water, adjust the pH to 7 with 5N aqueous sodium hydroxide solution, and then extract the water layer 3 times with 40ml of 1:1 mixed solvent of ethyl tetrahydrofuran acetate , combined the organic layers, dried over anhydrous magnesium sulfate, filtered, and concentrated to dryness under reduced pressure at 40°C to obtain 4.9 g of white crystals. 60ml (volume ratio 7 / 3) mixed solution of isopropyl ether and ethyl acetate was used for recrystallization to obtain 3.7g of white crystals of compound 2, Mp101°C.
[...
Embodiment 2
[0027] Embodiment 2: the synthesis of compound (Ⅳ)
[0028] Under nitrogen protection, 8g (14.6mmol) 10-deacetylbaccatin III (II) was added in 100ml of anhydrous chloroform, stirred and dissolved, then added 2.86g (16.0mmol) N, N'-thiocarbonyldiimidazole ( III), stirring reaction at room temperature, the reaction system is bright yellow and transparent, continue to react for 1 hour, the reaction solution is washed with water and saturated sodium chloride, MgSO 4 Dry, filter, concentrate to dryness under reduced pressure at 55°C, and crystallize with ethyl acetate / n-hexane to obtain 8.6 g of white solid compound (IV). Mp145-148℃
Embodiment 3
[0029] Embodiment 3: the synthesis of compound (Ⅵ)
[0030] Under the protection of nitrogen, put 8.6g (13.1mmol) of the obtained compound (IV) into a three-necked flask, add 100ml of anhydrous pyridine and stir to dissolve, cool down to 10°C, and add trichloroethoxycarbonyl chloride (Ⅴ) dropwise under stirring 3.2g (15mmol) was diluted with 5ml of toluene, naturally warmed to room temperature, then stirred and reacted at room temperature for 1 hour, cooled, 100ml of n-hexane was added to the reaction solution, left overnight, and filtered to obtain 9.2g of white crystalline compound (VI).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com