Mezlocillin sodium trihydrate and preparation method thereof
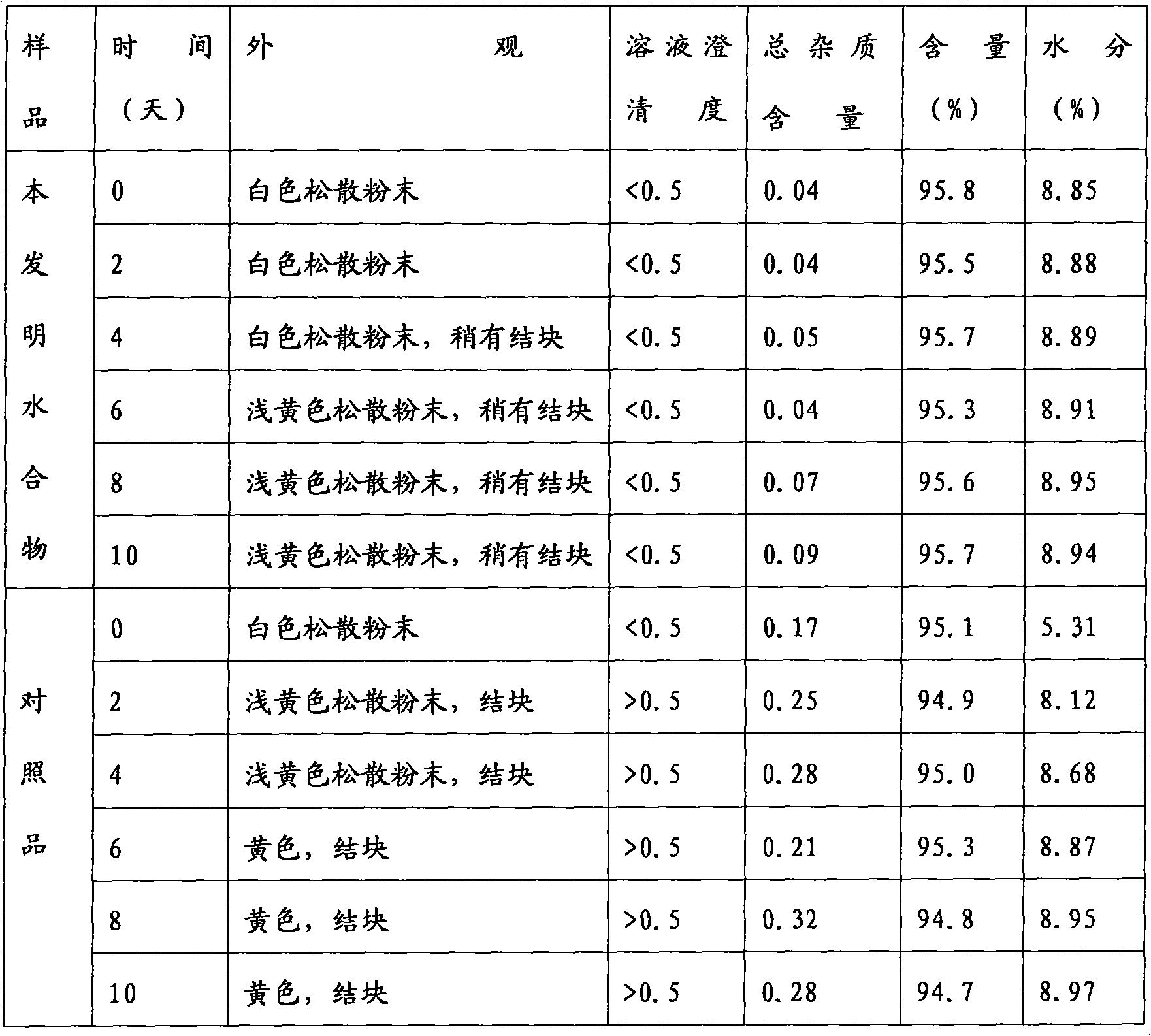
A technology of mezlocillin sodium trihydrate and mezlocillin sodium is applied in the directions of active ingredients of heterocyclic compounds, organic chemistry, antibacterial drugs, etc. Good fluidity and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] The preparation method of mezlocillin sodium trihydrate: comprise the steps:
[0037] (1), with 5-10 parts of mezlocillin sodium raw material drug, dissolve with the water of 10-20 parts, consumption is weight part;
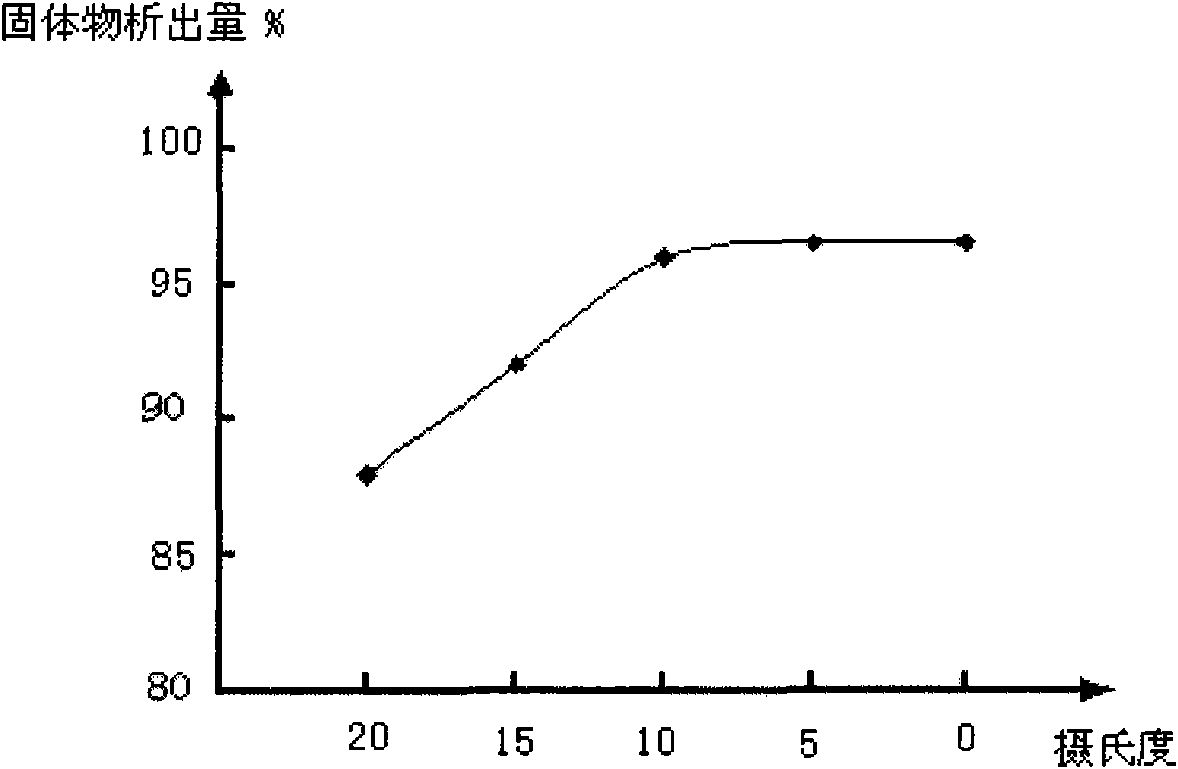
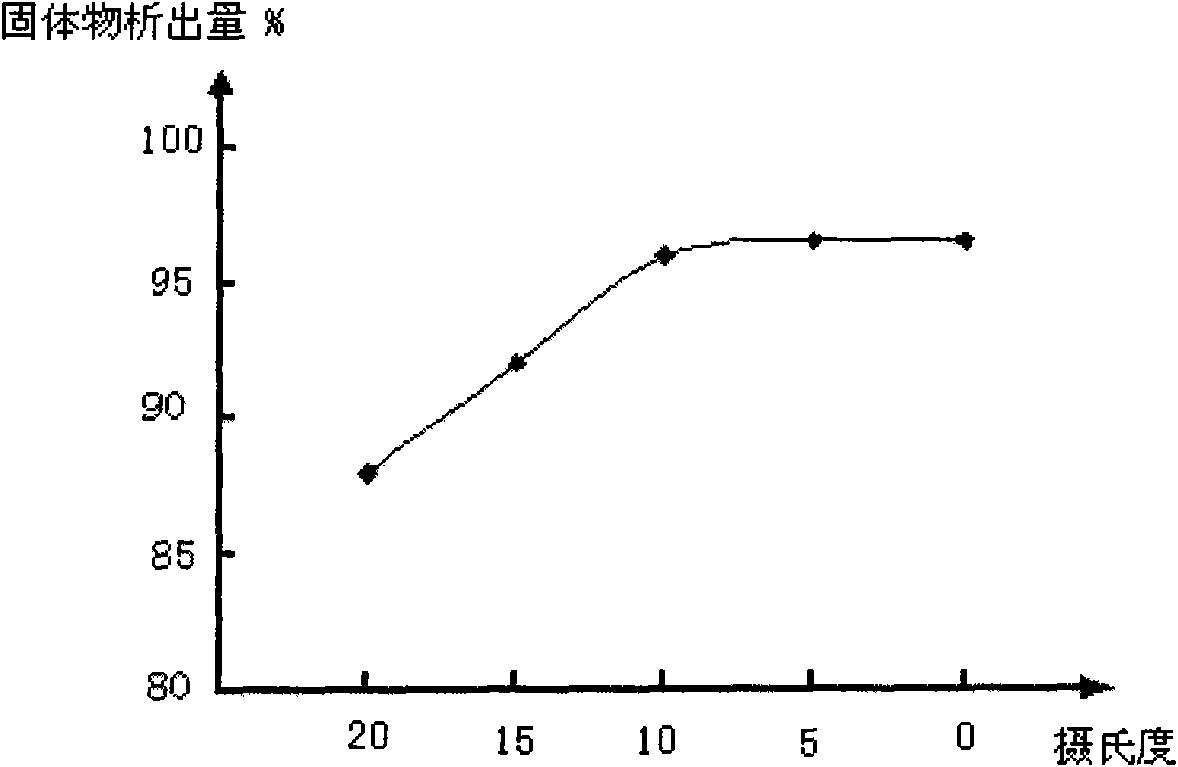
[0038] (2) Put the dissolving liquid into the crystallizer, slowly (1-10mL / min) add 60-90 parts by weight of eluting agent, cool to 2-8°C, keep the temperature for 2-5 hours, and wait for the solid matter to precipitate, Filter to get solids;
[0039] (3), the solid matter is rinsed with 3-5 parts of dissolving agent, and dried under ventilated or vacuum condition, drying time 3~5 hours, drying temperature 10~50 ℃, get mezlocillin sodium trihydrate ( Its function is to remove volatile impurities and moisture).
[0040] The solvent is one or more of acetone, dichloromethane, chloroform, ethanol, isopropanol, n-propanol, ether, methyl acetate, ethyl acetate, and butyl acetate.
[0041] The cooling temperature in the (2) step is 8° C. (preferred temperatur...
Embodiment 1
[0043] Weigh 10 g of mezlocillin sodium raw material, place in a 200 ml beaker, add 20 g of water to dissolve. Slowly add (8 mL / min) 90 g of isopropanol, stir while adding, control the temperature to 8° C., and keep the temperature constant for 2 hours.
[0044] Filter with suction, wash the filter cake with 5 g of isopropanol, and dry in vacuum at 10° C. for 5 hours to obtain mezlocillin sodium trihydrate.
Embodiment 2
[0046] Weigh 5 g of mezlocillin sodium raw material, place in a 200 ml beaker, add 10 g of water to dissolve. Slowly add (2 mL / min) 60 g of eluting agent, stir while adding, eluting agent is 25 g of acetone, 35 g of ethyl acetate, control the temperature to 2°C, and keep the temperature constant for 5 hours.
[0047] Suction filtration, wash the filter cake with 3 g of acetone, and vacuum-dry at 50° C. for 3 hours to obtain mezlocillin sodium trihydrate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com