Method for separating and purifying scallop phenol oxidase
A scallop phenol oxidase, separation and purification technology, applied in the field of shellfish immunology, can solve problems such as no relevant reports, and achieve the effects of improving purification efficiency, simple equipment requirements, and short time consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
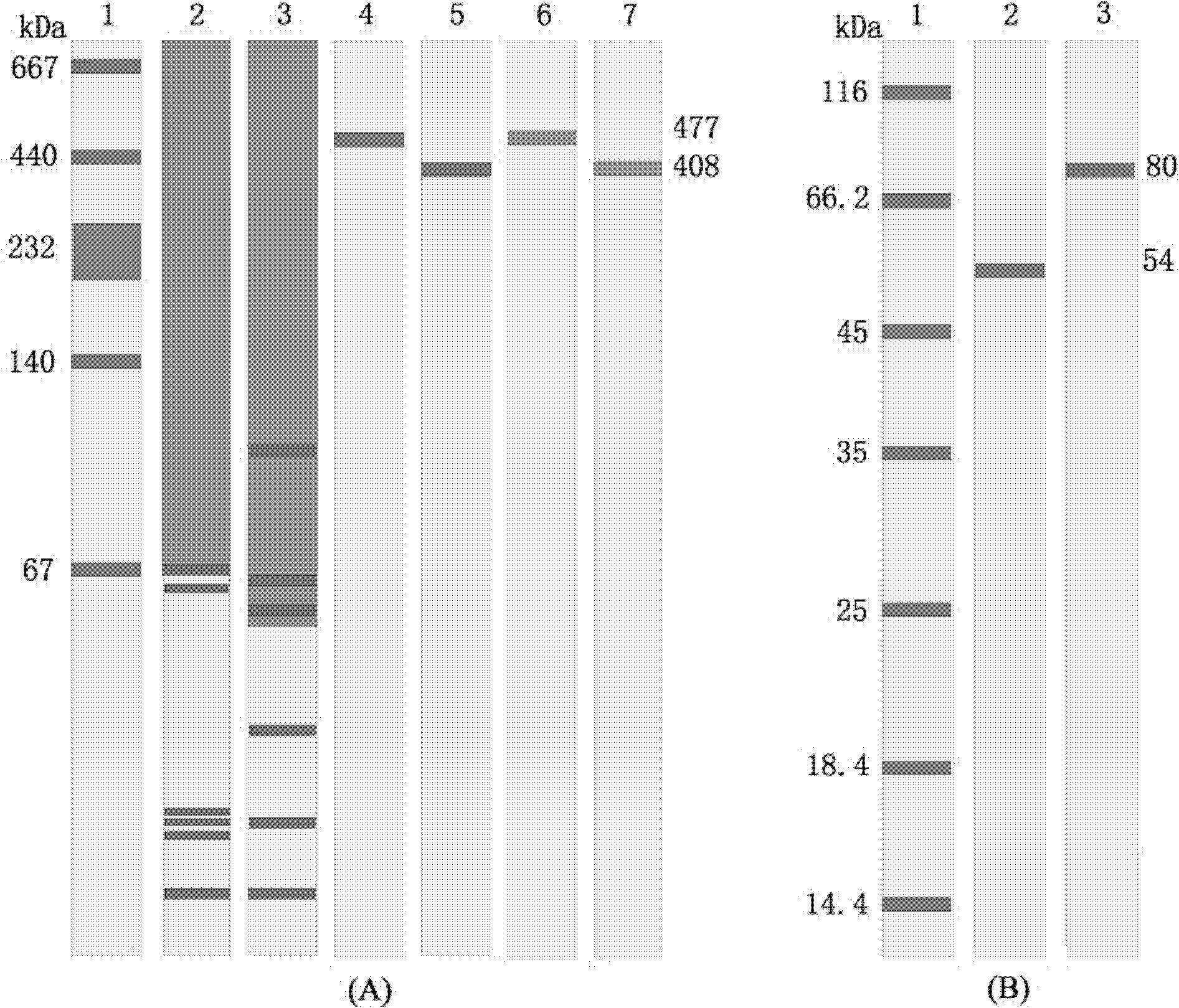
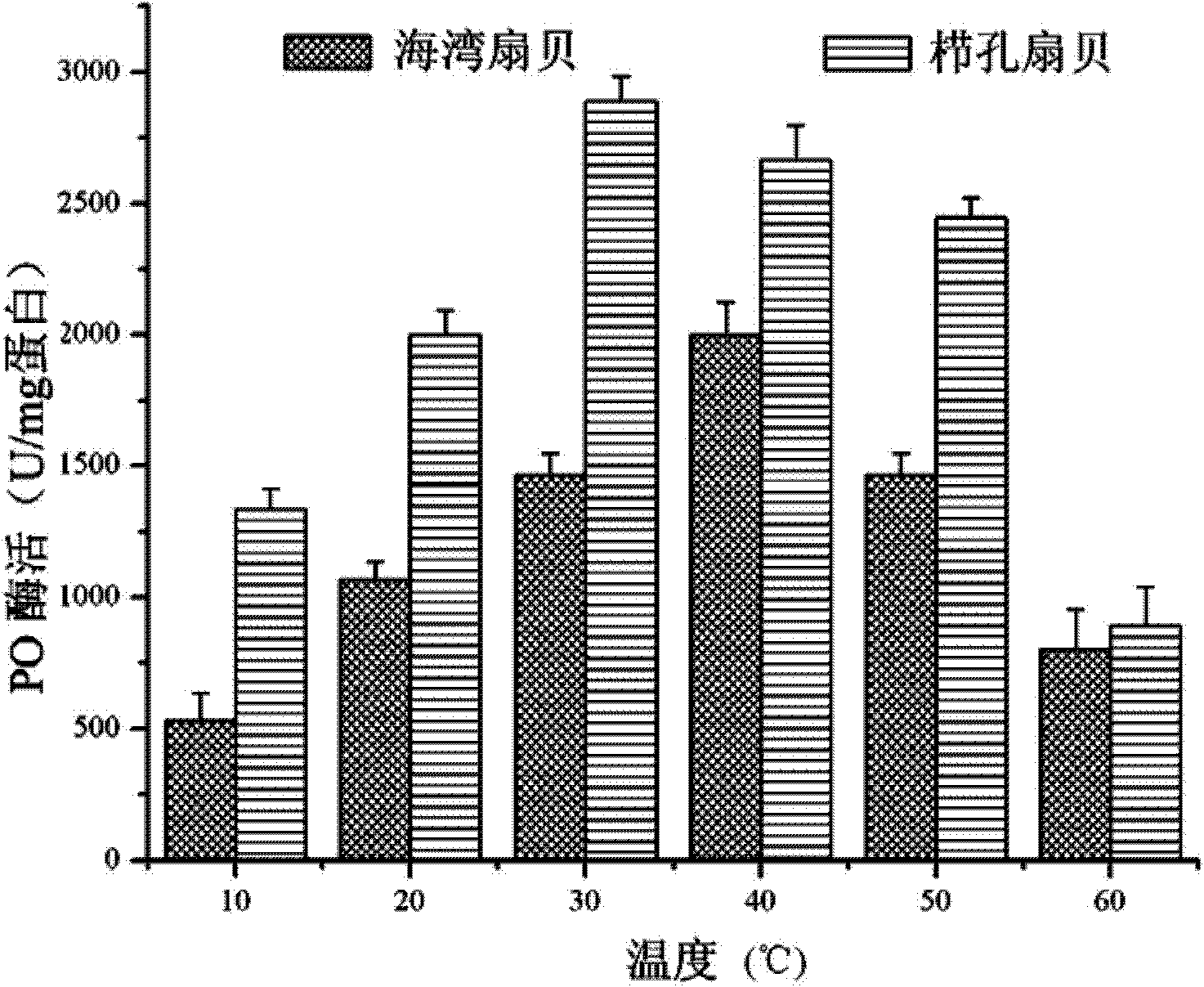
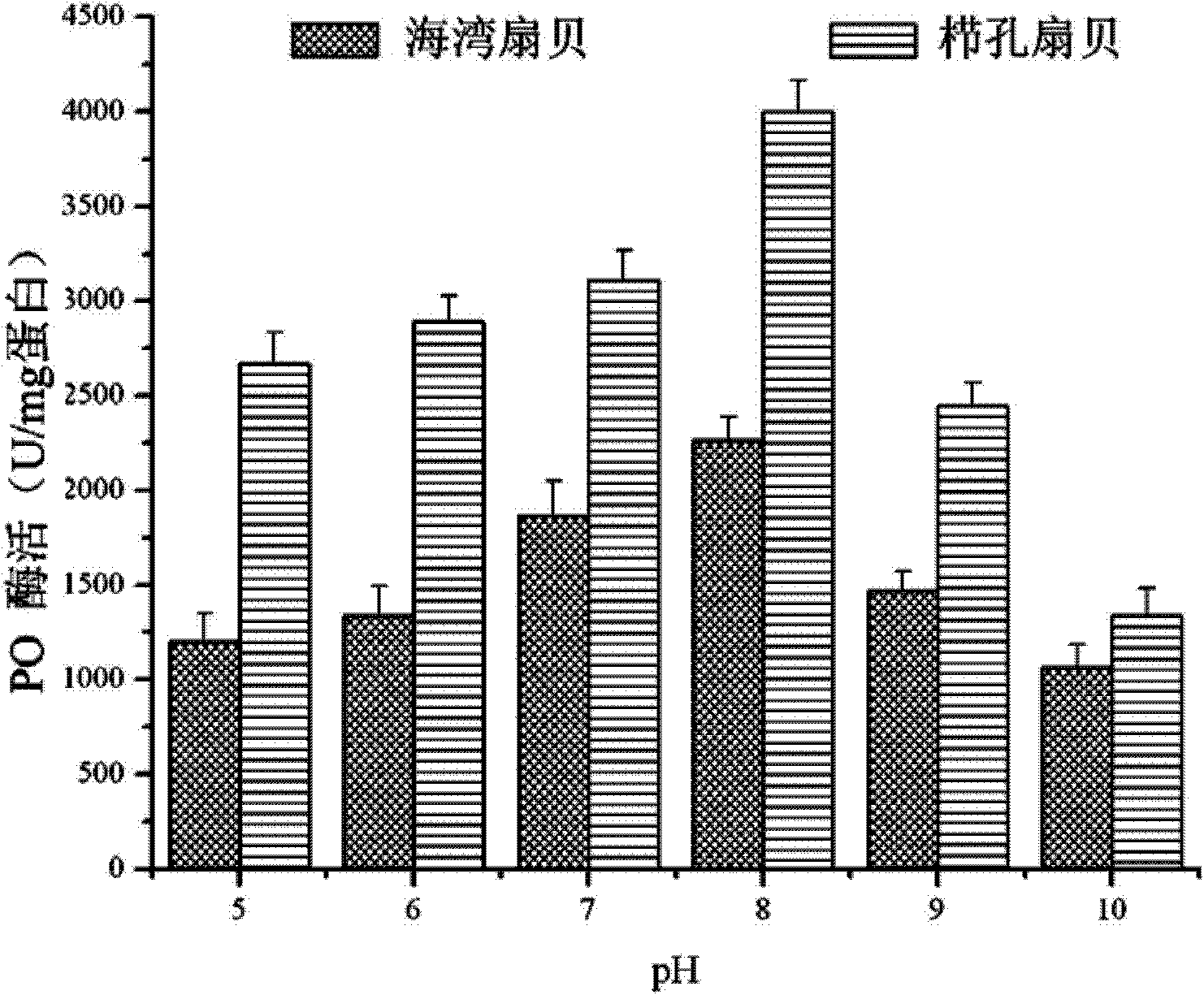
[0024] Example 1: Separation and purification of bay scallop (Argopectenirradians) PO by continuous gradient non-denaturing electrophoresis-specific substrate color development-electroelution
[0025] (1) Prepare the supernatant of blood cell lysate
[0026] ①Use a sterilized syringe to collect blood from the adductor muscle of bay scallop, centrifuge (4°C, 3000rpm, 15min), collect the blood cell pellet, and use 1.5ml of 20mM Tris- Resuspend in HCl buffer (pH 7.0);
[0027] ② Disrupt the blood cell suspension with an ultrasonic disruptor at 4°C, centrifuge (4°C, 14,000 rpm, 30 min), and take the supernatant, which is the supernatant of the blood cell disruption solution.
[0028] (2) Continuous gradient non-denaturing electrophoresis using the supernatant of blood cell lysate as a sample
[0029] ① Prepare pH 8.8 Tris-Glycin non-denaturing electrophoresis buffer (6.06g Tris, 28.82g Glycin, 2L DW), and pre-cool to 4°C.
[0030] ② Use a constant flow pump to prepare a continu...
example 2
[0072] Example 2: Separation and purification of Chlamysfarreri PO by continuous gradient non-denaturing electrophoresis-specific substrate color development-electroelution
[0073] (1) Prepare the supernatant of blood cell lysate
[0074] ①Use a sterilized syringe to take blood from the adductor muscle of Chlamys farreri, centrifuge (4°C, 3000rpm, 15min) to collect the blood cell pellet, and use 1.5ml of 20mM Tris pre-cooled to 4°C for the blood cell pellet obtained by centrifuging about 100ml of hemolymph -HCl buffer (pH 7.0) resuspended;
[0075] ② Disrupt the blood cell suspension with an ultrasonic disruptor at 4°C, centrifuge (4°C, 14,000 rpm, 30 min), and take the supernatant, which is the supernatant of the blood cell disruption solution.
[0076] (2) Continuous gradient non-denaturing electrophoresis using the supernatant of blood cell lysate as a sample
[0077] Same as step (2) in Example 1, replace the sample with the supernatant of blood cell disruption liquid o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Michaelis constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com