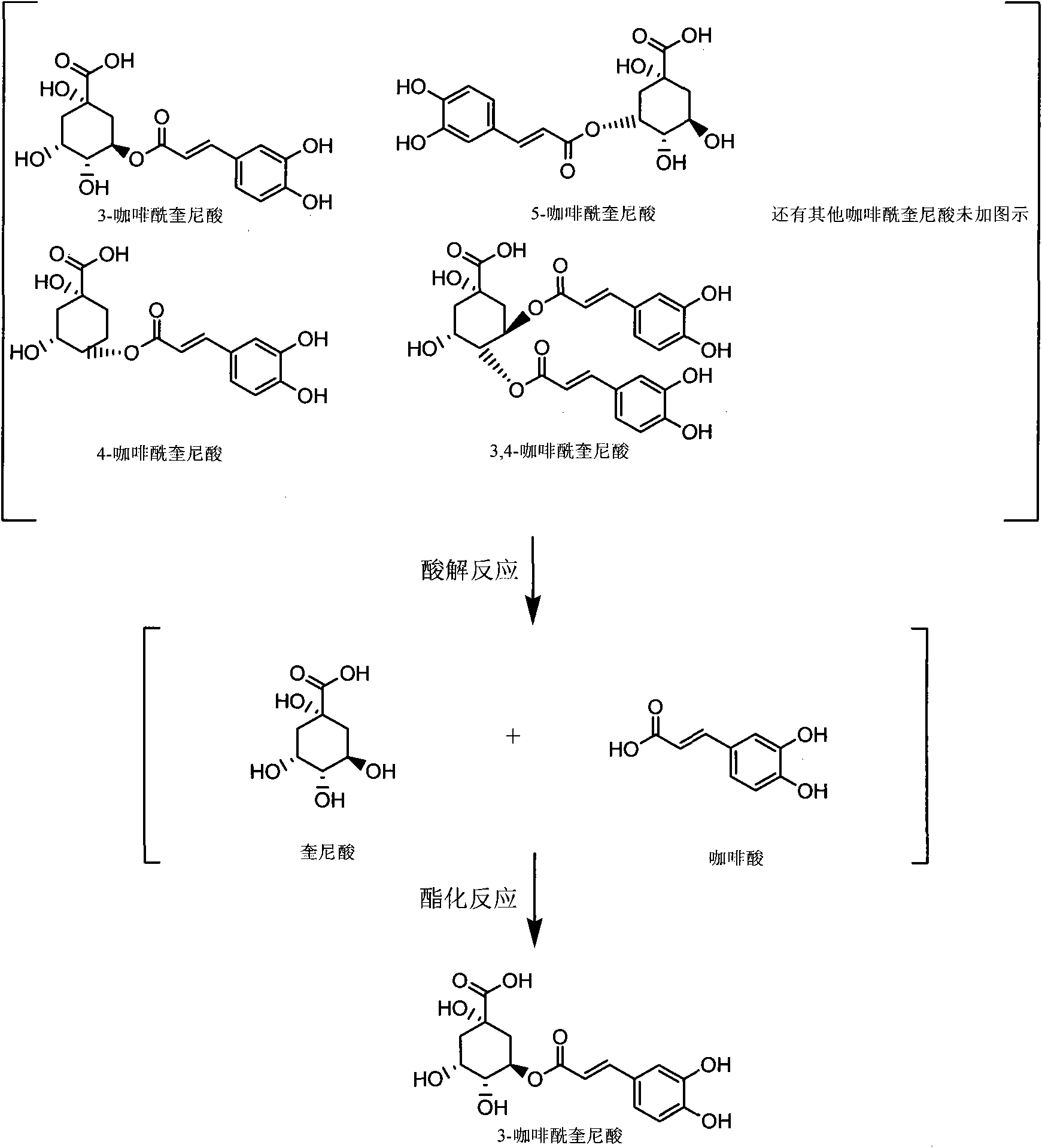
Method for preparing and partially-synthesizing chlorogenic acid from processing residual liquid of aqua lonicerae foliae
A technology for honeysuckle dew and chlorogenic acid, which is applied in the field of chemical substances or pharmaceutical manufacturing, can solve the problems of difficult to scale up production of separation technology, low yield of chlorogenic acid, low utilization rate of raw materials, etc., and achieves simple process and improved utilization of raw materials. rate, the effect of solving the low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] (1) Precipitation step
[0033] Get 100ml honeysuckle dew processing residual liquid, add 2% trichloroacetic acid aqueous solution (V / V) 10ml, add volume and honeysuckle dew processing residual liquid volume ratio is 1: 10 (V / V), add hexadecyl trimethyl Ammonium bromide (CTAB) 2.0g, the ratio of its added quality to the volume of honeysuckle dew processing raffinate is 0.02g / ml (mass to volume ratio), after stirring and reacting at 60°C for 60min, leave it standing at 4°C for 24 hours, filter the The obtained filtrate was concentrated to 10ml, and its volume was 1 / 10 of the volume of the honeysuckle dew processing residual liquid to obtain 10ml of precipitated liquid.
[0034] (2) Macroporous adsorption resin separation step
[0035] After the precipitated solution obtained is loaded onto the AB-8 macroporous adsorption resin, it is eluted with 5% ethanol aqueous solution (V / V), and the flow rate is 3 column volumes per hour, discarding the eluent, and then using 60% ...
Embodiment 2
[0045] (1) Precipitation step
[0046] Get 200ml honeysuckle dew processing residual liquid, add 5% trichloroacetic acid aqueous solution (V / V) 10ml, add volume and honeysuckle dew processing residual liquid volume ratio is 1: 20 (V / V), add hexadecane hydroxide simultaneously Trimethylamine bromide (CTA-OH) 16.0g, the ratio of its added mass to the volume of honeysuckle dew processing residual liquid is 0.08g / ml (mass volume ratio), stirred and reacted at 20°C for 40min, then stood at 25°C for 8 hours , the filtrate obtained after filtering is concentrated to 10ml, its volume is 1 / 20 of the volume of the honeysuckle dew processing residual liquid, and 10ml of precipitation solution is obtained.
[0047] (2) Macroporous adsorption resin separation step
[0048] After loading the obtained precipitate solution onto the XDA-1 macroporous adsorption resin, elute it with 20% ethanol aqueous solution (V / V), the flow rate is 1 column volume per hour, discard the eluate, and then use ...
Embodiment 3
[0058] (1) Precipitation step
[0059] Get 300ml honeysuckle dew processing raffinate, add 3% trichloroacetic acid aqueous solution (V / V) 20ml, add volume and honeysuckle dew processing raffinate volume ratio is 1: 15 (V / V), add hexadecylpyridinium simultaneously (CP-OH) 18.0g, the ratio of its added mass to the volume of honeysuckle dew processing residual liquid is 0.06g / ml (mass volume ratio), stirred and reacted at 40°C for 20min, left standing at 10°C for 18 hours, filtered to obtain The filtrate is concentrated to 10ml, and its volume is 1 / 30 of the volume of the honeysuckle dew processing residual liquid to obtain 10ml of precipitated liquid.
[0060] (2) Macroporous adsorption resin separation step
[0061] After loading the obtained precipitate solution onto D101 macroporous adsorption resin, elute with 10% ethanol aqueous solution (V / V), the flow rate is 2 column volumes per hour, discard the eluate, and then use 30% ethanol The aqueous solution (V / V) was eluted at...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com