Prasugrel intermediate and preparation method thereof
A technology of intermediates and reaction time, applied in the fields of pharmaceutical intermediates and their preparation, prasugrel intermediates and their preparations, can solve problems such as unfavorable industrialized production, high product purification difficulty, high environmental protection pressure, etc., and achieve production costs. Low, low environmental pressure, less effect of three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1: Alkylation reaction
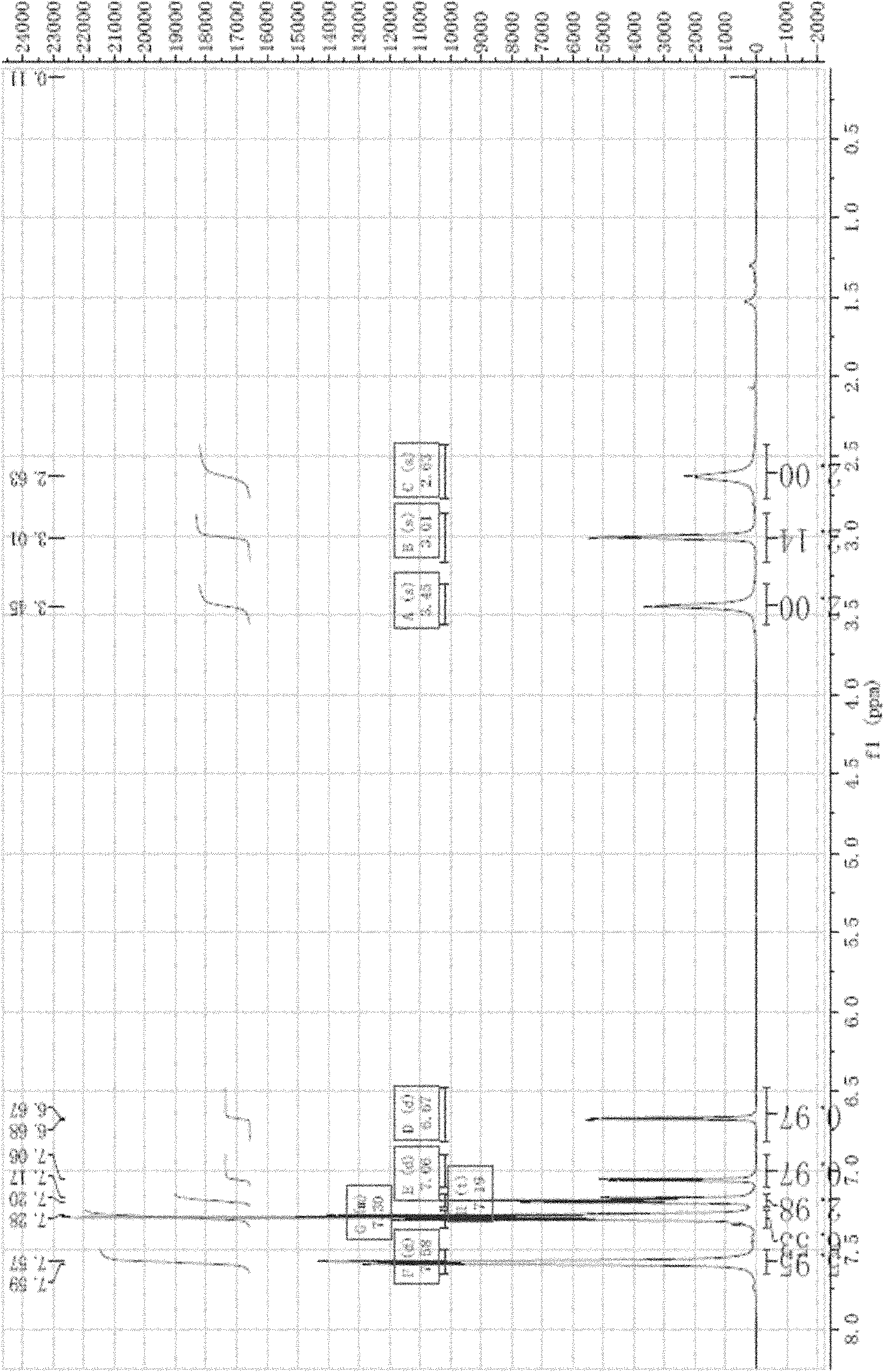
[0059] 4,5,6,7-Tetrahydrothiophene[3,2-c]pyridine hydrochloride (140.4g, 0.80mol) was dissolved in dichloromethane (500mL), added triethylamine 233.6mL (1.68mol) and stirred After 15 min, a solution of triphenylchloromethane (234.0 g, 0.84 mol) in dichloromethane (400 mL) was added dropwise at room temperature, and the drop was completed within 2 h. Stir at the same temperature for 8 hours, pour the reaction solution into 500 mL of water, separate the organic phase, wash 3 times with saturated brine, dry the organic phase with anhydrous magnesium sulfate, rotary evaporate under reduced pressure, and wash the residue with cold ethanol (100 mL) to obtain white dry 298.1 g of solid, yield 98%.
[0060] white solid: 1H NMR (δ, CDCl 3 ): 7.57~7.59(m, 6H), 7.25~7.35(m, 6H), 7.17~7.20(m, 3H), 7.06, (s, 1H), 6.68(d, 1H), 3.45(s, 2H) , 3.01(s, 2H), 2.63(s, 2H). According to the data analysis of nuclear magnetic resonance, it can be confir...
Embodiment 2
[0061] Embodiment 2: Alkylation reaction
[0062] 140.4g (0.8mol) of 4,5,6,7-tetrahydrothiophene[3,2-c]pyridine hydrochloride was dissolved in dichloromethane (500mL), and 231.8g (1.68mol) of solid potassium carbonate was added, and stirred After 15 minutes, a solution of 234.0 g (0.84 mol) of triphenylchloromethane in dichloromethane (400 mL) was added dropwise at room temperature, and the drop was completed within 2 hours. Raise the temperature to 60°C and stir for 8 hours. After the reaction is complete, cool the reaction solution to room temperature and pour it into 500 mL of water. Separate the organic phase and wash it with saturated brine for 3 times. Dry the organic phase with anhydrous magnesium sulfate. After washing with cold ethanol (100 mL), 282.7 g of a white dry solid was obtained, with a yield of 93%.
Embodiment 3
[0063] Embodiment 3: borylation reaction
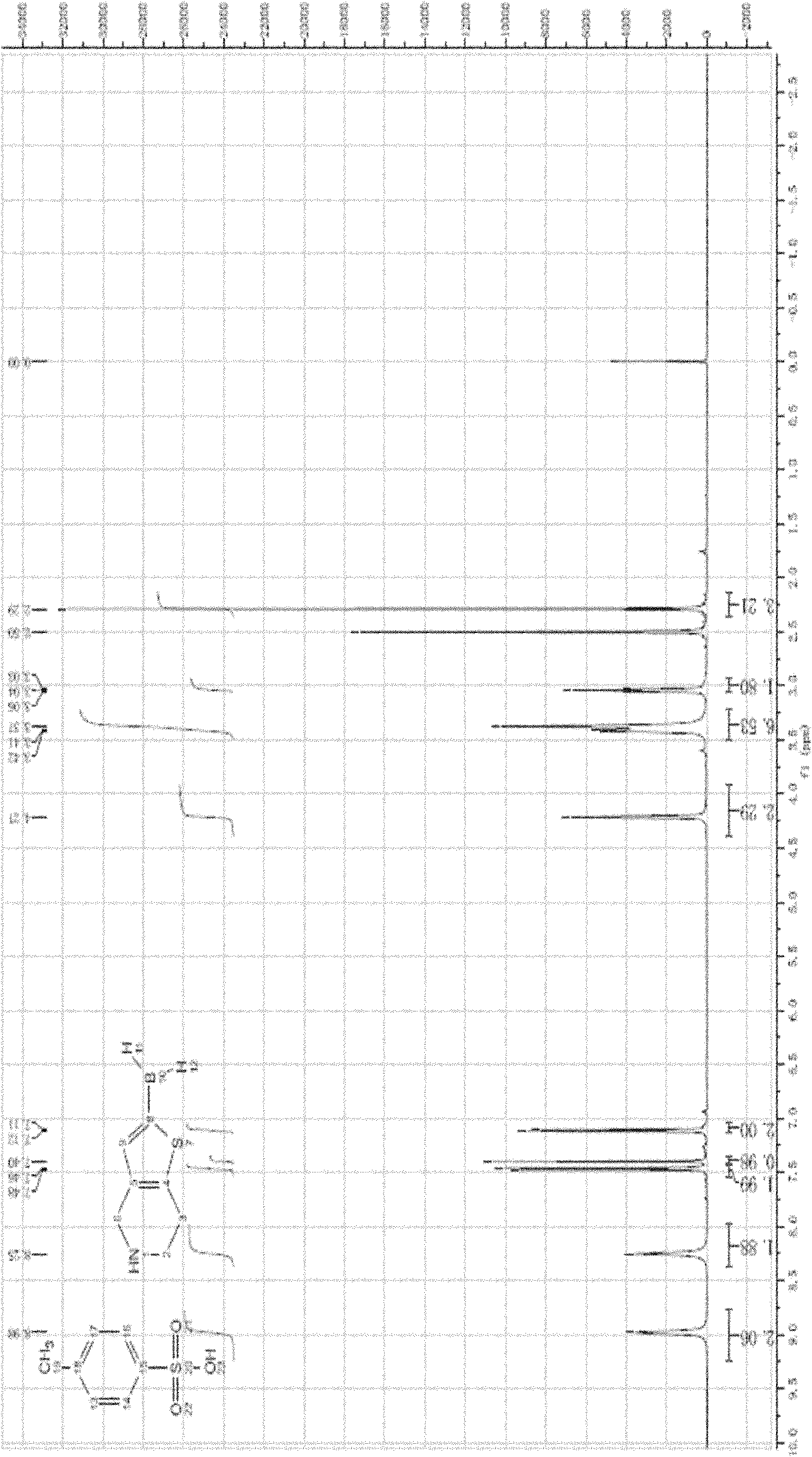
[0064] Take 19.0 g (0.05 mol) of the product (N-trityl-4,5,6,7-tetrahydrothiophene [3,2-c] pyridine) obtained in Example 1, dissolve it in 50 mL tetrahydrofuran (THF) 30 mL (0.075 mol) of n-butyllithium was added dropwise for 1 h at 0°C, under nitrogen protection, and stirred at the same temperature for 2 h. After cooling to -30°C, a mixture of 19 mL (0.075 mol) of trimethyl borate and THF (50 mL) was added dropwise, the addition was completed dropwise in half an hour, and the reaction was carried out at the same temperature for 1 h. Add 100 mL of ethyl acetate, wash three times with 100 mL of saturated sodium bicarbonate solution, wash and separate with 100 mL of saturated brine × 3, dry the organic phase with anhydrous magnesium sulfate, and concentrate by rotary evaporation at 40°C to obtain 20.2 g of the product, with a yield of 95% .
[0065] 1 H NMR (δ, CDCl 3 ): 7.55~7.57(m, 6H), 7.29~7.34(m, 7H), 7.09~7.20(m, 3H), 3.25~3.4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com