Preparation method of lithium iron phosphate cathode material for lithium ion battery
A lithium-ion battery and lithium iron phosphate technology, applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., can solve the problem of low mixing uniformity, achieve excellent electrical conductivity, good safety performance, and low production costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
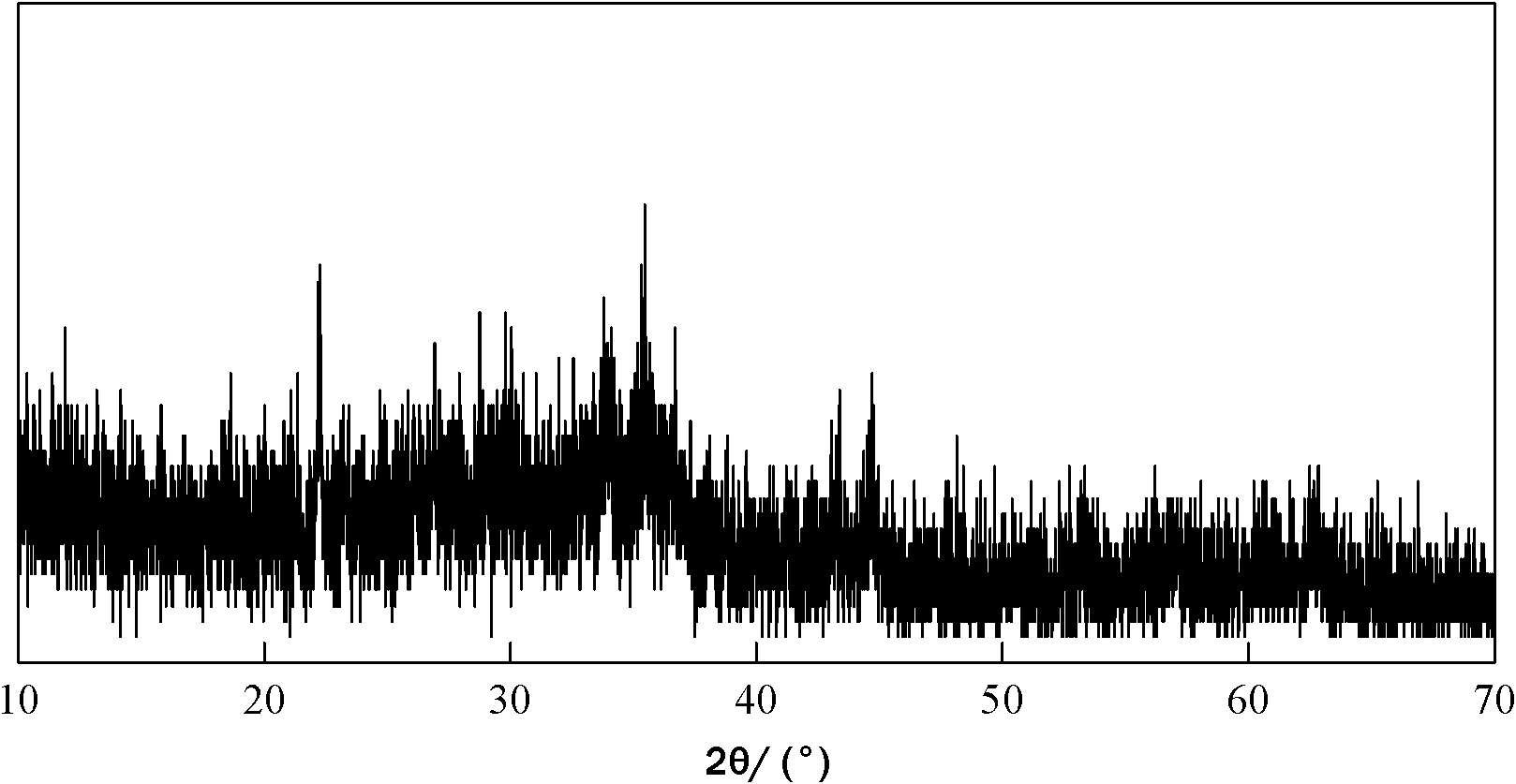
[0029] Mix the molar ratio of lithium hydroxide: ferrous oxalate: ammonium dihydrogen phosphate = 1.2: 1: 1, add 3% conductive carbon black by weight, mix and grind in a stirring ball mill for 10 minutes; add the grinding product to In the twin-screw extruder, the controlled extrusion conditions are: temperature 25°C, screw speed 500r / min, figure 1 It is the XRD pattern of the extrusion product A. It can be seen from the figure that a chemical reaction occurred between the raw materials during the reactive extrusion process, so an amorphous substance was formed; the extruded product was loaded into a porcelain boat and placed in an electric furnace. Under the nitrogen protection of 1 liter / min, the temperature was raised to 400°C at a rate of 10°C / min, and the temperature was maintained for 5 hours; then the temperature was raised to 700°C at a rate of 10°C / min, and the temperature was maintained for 12 hours; then cooled to room temperature with the furnace, The resulting pro...
Embodiment 2
[0032] Mix the molar ratio of lithium carbonate: ferrous oxalate: phosphorus pentoxide = 0.5: 1: 0.5, add 5% sucrose by weight, mix and grind for 30min in a stirring ball mill; add the ground product to a twin-screw extruder. The extrusion conditions were controlled as follows: the temperature was 120°C, the screw speed was 400r / min; the ground product was taken out, put into a porcelain boat, placed in an electric furnace, under the protection of argon gas with a flow rate of 5 liters / min, at 15 The temperature was raised to 800°C at a speed of °C / min, maintained at this temperature for 5 hours, and then cooled to room temperature with the furnace, and the obtained product was product C.
[0033] The battery was assembled according to the method of Example 1, and tested at room temperature and 0.5C current density. The results showed that its first discharge specific capacity was 147.4mAh / g, and its specific capacity after 10 cycles was 145.8mAh / g; its first charge-discharge c...
Embodiment 3
[0035] Mix the molar ratio of lithium hydroxide: niobium pentoxide: iron hydrogen phosphate = 0.9: 0.05: 1, add 1% phenolic resin by weight, mix and grind for 60min in a stirring ball mill; In the screw extruder, the extrusion conditions are controlled as follows: the temperature is 250 °C, the screw speed is 50 r / min; The temperature was raised to 600° C., the temperature was maintained for 20 hours, and then the temperature was lowered to room temperature at a cooling rate of 5° C. / min, and the obtained product was product D.
[0036] The battery was assembled according to the method of Example 1 and tested at room temperature and 1C current density. The results showed that the specific capacity of the first discharge was 143.6mAh / g, and the specific capacity after 20 cycles was 142.8mAh / g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com