Preparation method of p-hydroxystyrene and derivates thereof
A technology for p-hydroxystyrene and p-hydroxybenzaldehyde, which is applied in the field of preparation of p-hydroxystyrene and its derivatives, can solve the problems of heavy odor of pyridine, harsh conditions, and over 100% yield, and achieves alleviation of odor and recovery of The effect of low yield, reducing residual problems and improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
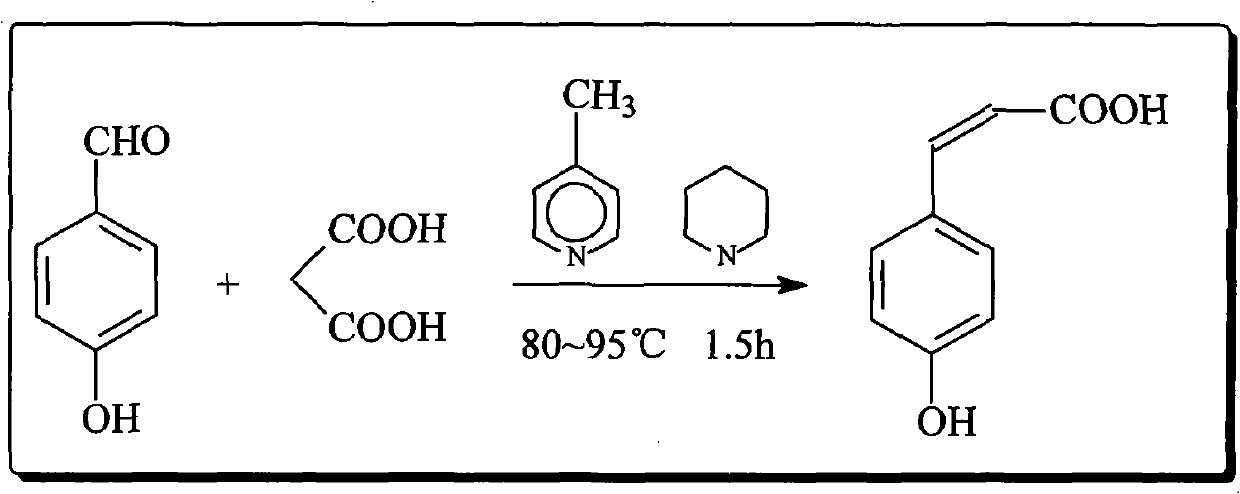
[0050] Weigh 10.0g of PHB, 12.8g of malonic acid, 17.0mL of p-picoline, and 2.0mL of piperidine, put them into a 100mL four-neck flask at one time, stir and heat to 85°C in an oil bath, keep the temperature for 1.5h, and pour into the feed solution after cooling Add 40mL of 20% potassium carbonate solution to the water layer and keep stirring, let it stand for layer separation, add 35mL dilute hydrochloric acid (prepared according to the volume ratio of dense fume hydrochloric acid and water 1:1) to the water layer to adjust the pH value to about 2 , filtered the precipitated solid, washed with water, and dried to obtain a crude product, which was recrystallized with distilled water to obtain 12.8 g of a light yellow solid, with a yield of about 95%.
Embodiment 2-5
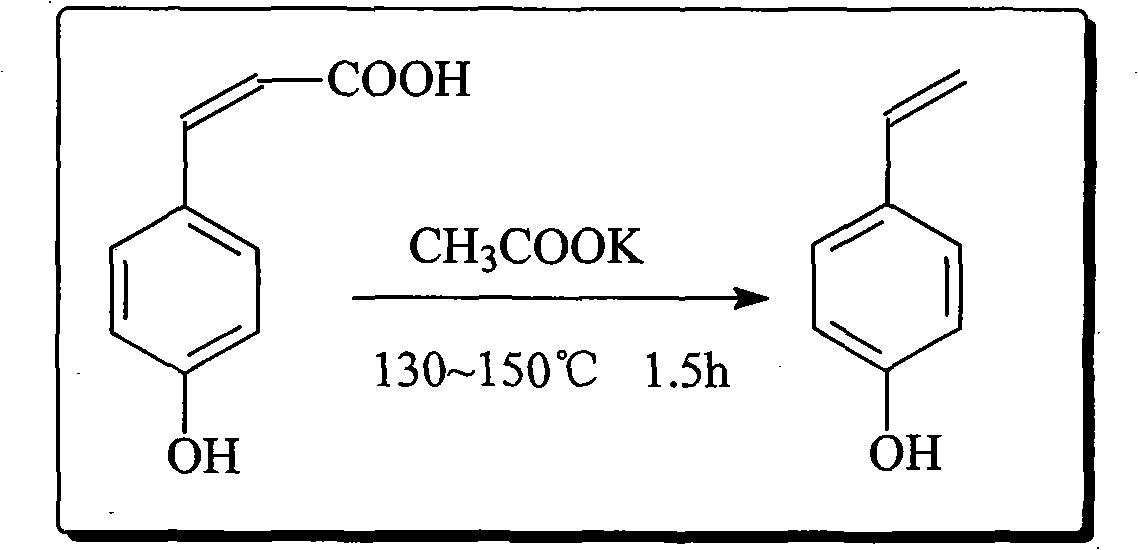
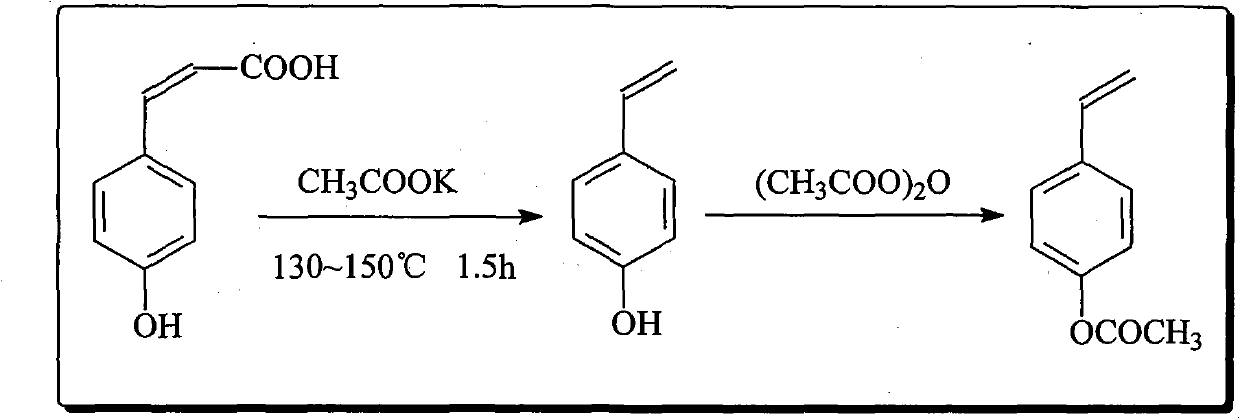
[0055] Embodiment 2-5: the preparation of PAS
[0056] Weigh 10.0g of PHCA, 0.05g of potassium acetate, 0.005g of hydroquinone, and 40mL of DMF, put them into a 100mL four-neck flask at one time, measure 12mL of acetic anhydride and pour them into the dropping funnel, stir and heat in an oil bath to 130°C, constant temperature 1.5 h, add acetic anhydride dropwise to it, continue to react for 1.5h after the dropwise addition, cool the feed liquid to room temperature, pour it into 100mL cold water, extract with the extract, dry over anhydrous magnesium sulfate, and remove the solvent by rotary evaporation to obtain pure PAS . The final product obtained by extraction with different extracts is as follows through gas phase detection:
[0057] Example
[0058] Taking cyclohexane extraction as an example, 9.2 g of light yellow liquid PAS was finally obtained, with a yield of about 93%.
Embodiment 6
[0059] Embodiment 6: the preparation of PTBOCS
[0060] Take 10.0 g of the final liquid of Example 1, 150 mL of dichloromethane, and 9.3 g of potassium tert-butoxide, and put them into a 250 mL four-neck flask at one time, measure 18 mL of di-tert-butyl dicarbonate and pour them into the dropping funnel, and stir mechanically at room temperature for 10 min. Add di-tert-butyl dicarbonate dropwise thereto, continue stirring for 2.0 h after the dropwise addition, wash the feed solution with water, dry over anhydrous magnesium sulfate, and rotary evaporate to obtain 16.0 g of light yellow liquid PTBOCS. The yield is about 87%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com