Method for preparing rod-like imprinted polymer capable of efficiently separating naringin in water phase
A technology for imprinting polymers and naringin, which is applied in the fields of alkali metal compounds, chemical instruments and methods, alkali metal oxides/hydroxides, etc., and can solve problems such as easy loss in screening treatment, cumbersome grinding of powder polymers, etc. , to achieve the effect of improving selective recognition, efficient separation, and increasing load capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
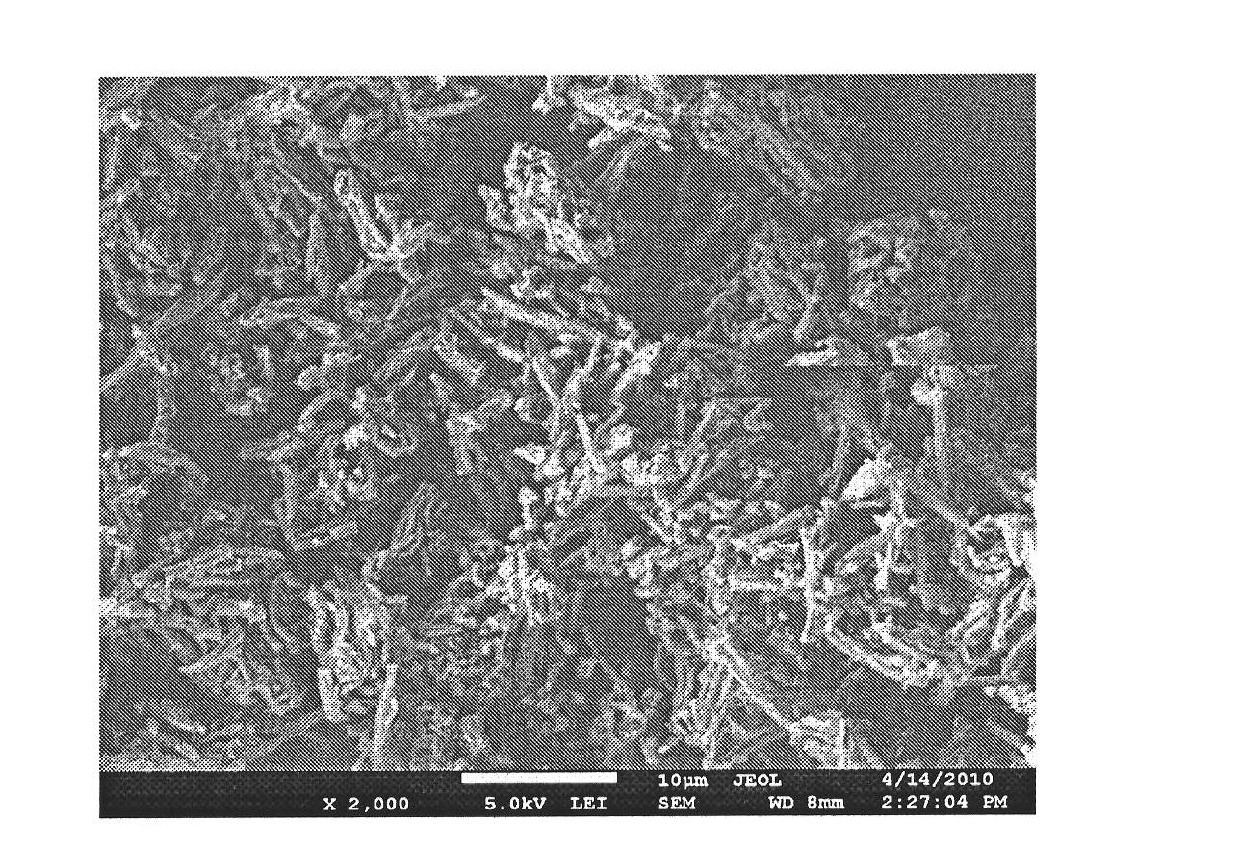
[0034] Dissolve 10g of β-CD and 3.1g of NG in an appropriate amount of 10% dimethyl sulfoxide aqueous solution at 30°C, then add 7mL of cross-linking agent hexamethylene diisocyanate (HMDI) dropwise while stirring, for 30min Finish. After keeping the reaction at this temperature for 3 h, add 10 g of liquid paraffin containing 0.8 g of emulsifier (Tween 20:Span 80=1:3), stir rapidly for 10 min and then raise the temperature to 75°C, while reducing the stirring speed and stirring at a constant speed Reaction 10h. After the reaction, the product was filtered and washed thoroughly with methanol, distilled water and acetone in order to remove various impurities. Finally, the NG absorption peak at 283nm was detected in the washing solution with a UV photometer. After washing, the product was vacuum-dried to constant weight to obtain a rod-shaped molecularly imprinted cyclodextrin polymer. The rod-shaped molecularly imprinted cyclodextrin polymer has a large specific surface area ...
Embodiment 2
[0036] Dissolve 4g of hydroxypropyl cyclodextrin and 4.15g of naringin dihydrochalcone in an appropriate amount of 30% N,N-dimethylacetamide aqueous solution at 30°C, and then add 10mL of it dropwise while stirring. Joint agent lysine diisocyanate, 20min to complete. After keeping the reaction at 30°C for 5h, add 15g of liquid paraffin containing 0.3g of emulsifier (Tween 80:Span 40=1:5), stir rapidly for 10min and then raise the temperature to 60°C, while reducing the stirring speed and stirring at a constant speed Reaction 8h. After the reaction, the product was filtered and washed thoroughly with methanol, distilled water and acetone in order to remove various impurities. Finally, the NG absorption peak at 283nm was detected in the washing solution with a UV photometer. After washing, the product was vacuum-dried to constant weight to obtain a rod-shaped molecularly imprinted cyclodextrin polymer.
Embodiment 3
[0038]Dissolve 2g of γ-cyclodextrin and 0.5g of quercetin in an appropriate amount of 20% N,N dimethylacetamide aqueous solution at 30°C, then add 6mL of cross-linking agent toluene diisocyanate dropwise while stirring, and complete in 20 minutes . After keeping the reaction at this temperature for 1 h, add 10 g of aviation kerosene containing 0.4 g of emulsifier (Tween 20: Span 20 = 1: 10), stir rapidly for 20 min and then heat up to 25 ° C, while reducing the stirring speed and stirring at a constant speed Reaction 7h. After the reaction, the product was filtered and washed thoroughly with methanol, distilled water and acetone in order to remove various impurities. Finally, the NG absorption peak at 283nm was detected in the washing solution with a UV photometer. After washing, the product was vacuum-dried to constant weight to obtain a rod-shaped molecularly imprinted cyclodextrin polymer.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com