Method for preparing nanocrystalline lithium iron phosphate powder by adopting iron hydroxide colloid
A technology of crystalline lithium iron phosphate and iron hydroxide glue, which is applied in the field of preparation of positive electrode materials for lithium-ion power batteries, can solve problems such as difficult industrialization, large secondary aggregates, and long synthesis cycle, and achieve high specific capacity, Strong specific surface area, high and low temperature effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
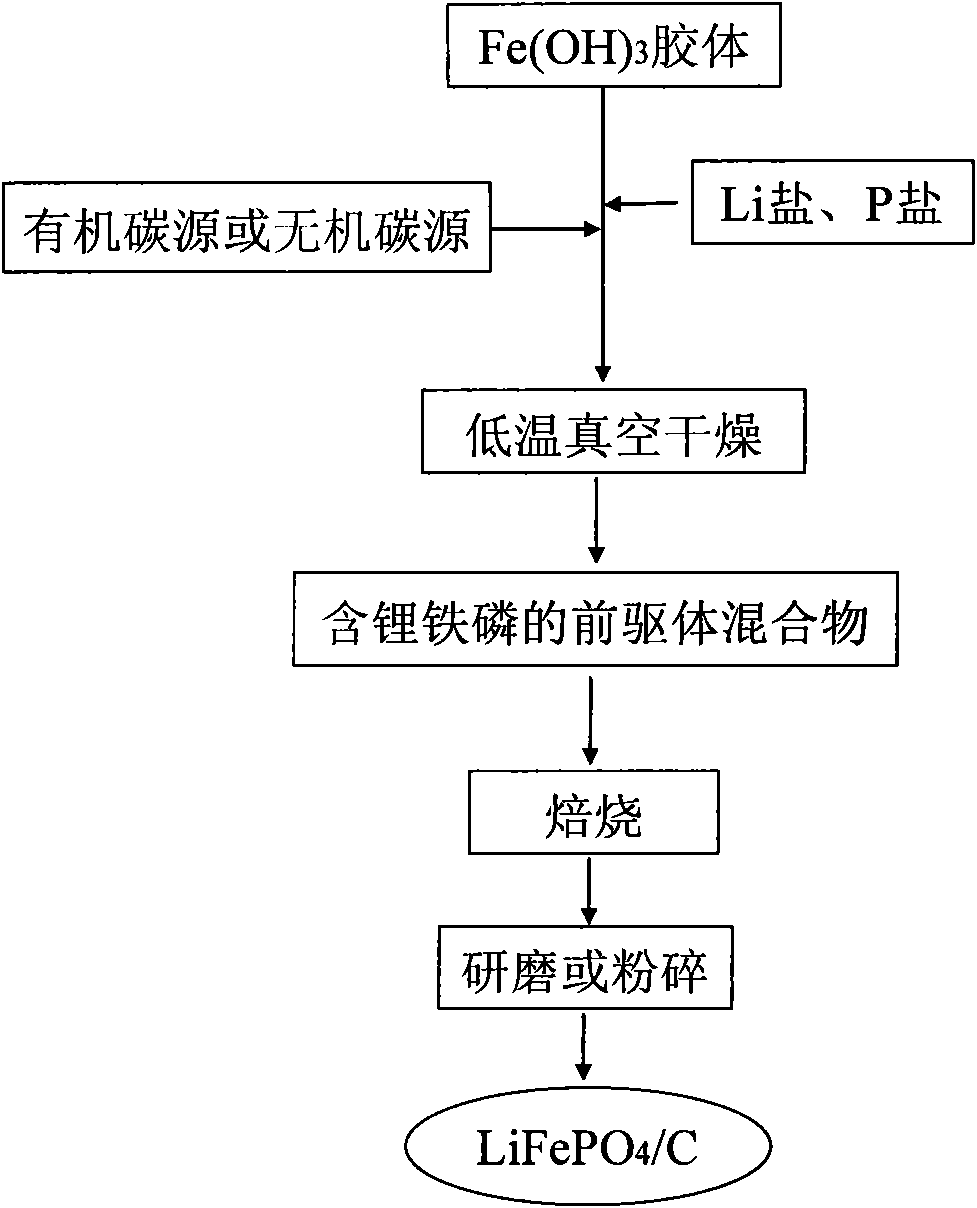
[0019] 85.61gFeCl 3 ·6H 2 O was dissolved in 93.26 mL of deionized water to form FeCl 3 saturated solution, and dropwise into boiling water to prepare fresh reddish-brown Fe(OH) 3 Colloids are obtained through the verification of Tyndall's phenomenon. Add 32.94gLiH to the above colloid 2 PO 4 At the same time, 19.60 g of starch was added as an organic carbon source, and the mixture was mixed evenly by rapid stirring. Then, the vacuum degree was kept at 0.09 MPa at 50 ° C, and dried for 24 hours to obtain the precursor powder. The precursor was placed in a nitrogen-protected muffle furnace, heated to 500 °C at a rate of 1 °C / min, kept for 24 hours, and then naturally cooled to room temperature. After taking it out, it was ground or pulverized to obtain LiFePO 4 / C powder material.
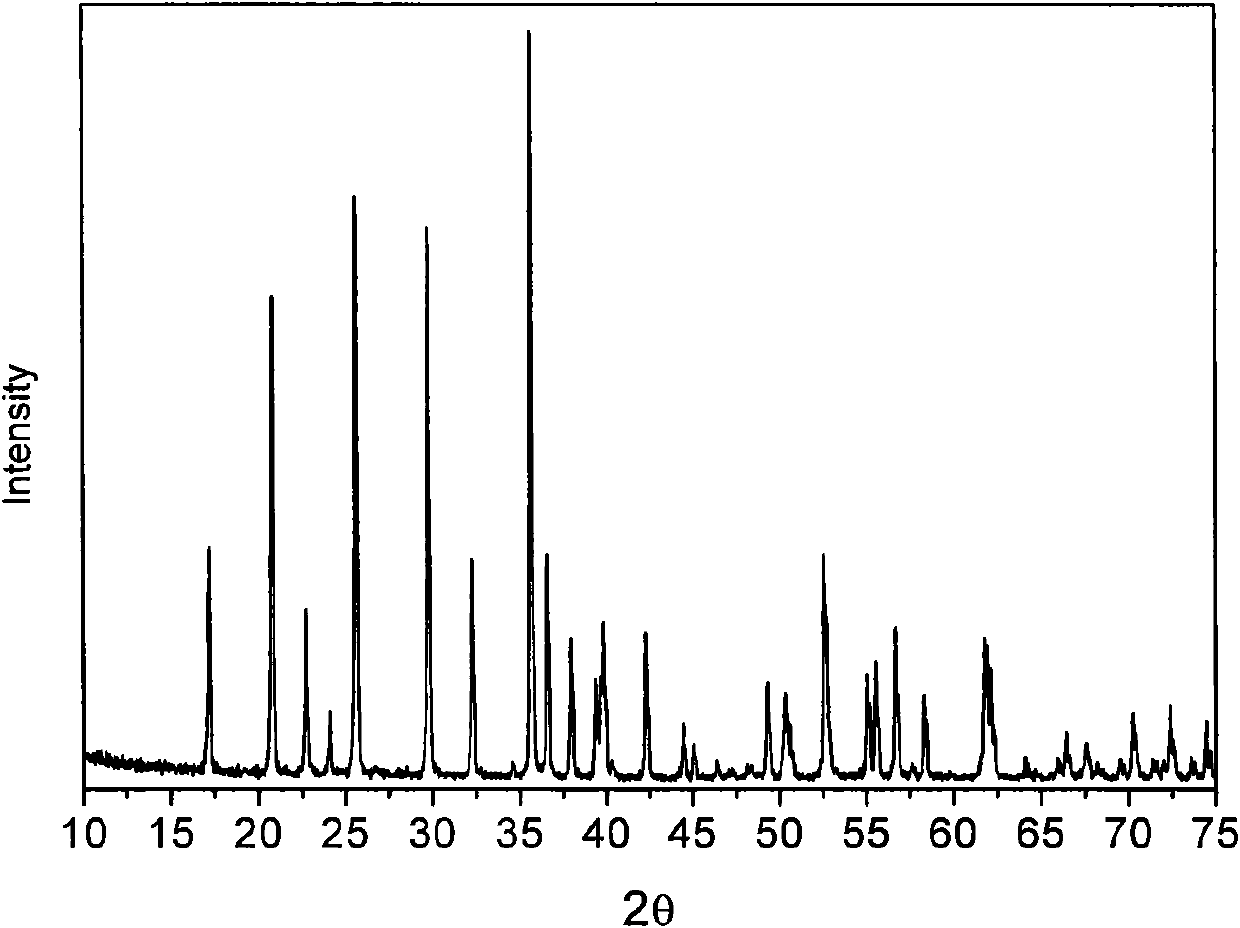
[0020] The XRD of the product that present embodiment obtains is as figure 2 As shown, it can be seen that a pure-phase olivine-type lithium iron phosphate material was synthesized. The ele...
Embodiment 2
[0023] 85.61gFeCl 3 ·6H 2 O was dissolved in 93.26 mL of deionized water to form FeCl 3 saturated solution, then dropwise into deionized water while adding a small amount of 10% NH 3 ·H 2 O, forming fresh reddish-brown Fe(OH) 3 Colloids are obtained through the verification of Tyndall's phenomenon. Add 12.89gLi to the above colloid 2 CO 3 Solid nanoscale powder and contains 36.43gNH 4 h 2 PO 4 At the same time, 21.40 g of glucose was added as an organic carbon source, stirred rapidly and evenly, and then kept at 100 °C with a vacuum degree of 0.02 MPa, and dried for 5 h to obtain a precursor powder. Put the precursor into a muffle furnace, heat up to 800°C at a rate of 0.5°C / min, keep it warm for 2 hours, and then cool it down to room temperature naturally. After taking it out, it is ground or pulverized to obtain LiFePO 4 / C powder material.
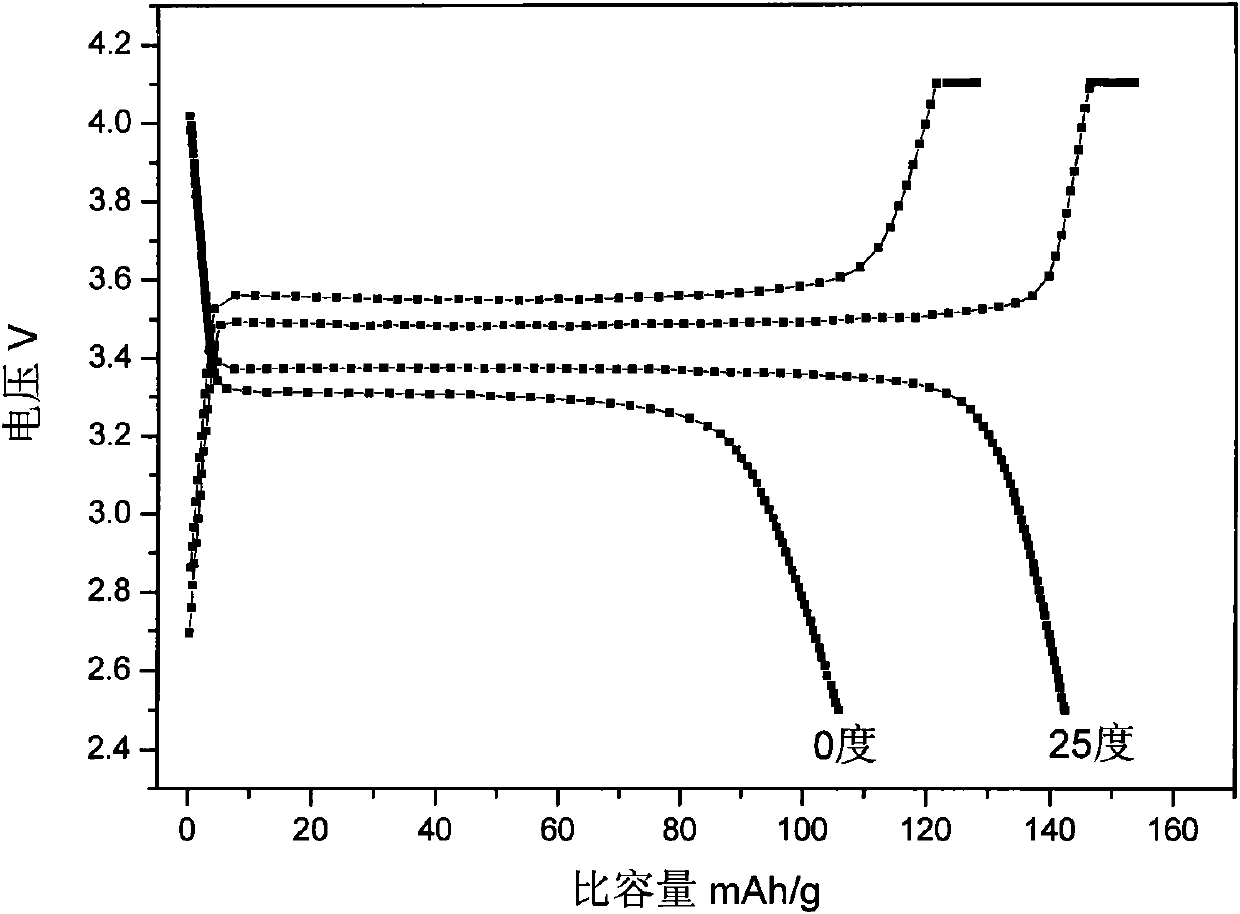
[0024]Test the electrical properties of the above positive electrode materials, the 0.2C discharge specific capacity at 25 ...
Embodiment 3
[0026] 128.03gFe(NO 3 ) 3 9H 2 O was dissolved in 114.31 mL of deionized water to form a saturated solution, and then dropped into deionized water drop by drop while adding a small amount of 10% NH 3 ·H 2 O, forming fresh reddish-brown Fe(OH) 3 Colloids are obtained through the verification of Tyndall's phenomenon. Add a solution containing 7.60g LiOH and 36.55mLH to the above colloid 3 PO 4 (85% w.t.) solution, while adding 19.60 g of starch as an organic carbon source, stirring quickly and uniformly, then keeping the vacuum at 0.08 MPa at 70 ° C, and drying for 15 h to obtain a precursor powder. Put the precursor into a muffle furnace, heat up to 650°C at a rate of 1.5°C / min, keep it warm for 10 hours, and then cool it down to room temperature naturally. After taking it out, it is ground or pulverized to obtain LiFePO 4 / C powder material.
[0027] Test the electrical performance of the above positive electrode material, the 0.2C discharge specific capacity at 25 deg...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Discharge specific capacity | aaaaa | aaaaa |
| Discharge specific capacity | aaaaa | aaaaa |
| Discharge specific capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com