High-temperature-resistant transaldolase and preparation method thereof
A transaldolase, high-temperature-resistant technology, applied in the fields of botanical equipment and methods, biochemical equipment and methods, transferase, etc., can solve problems such as low activity and achieve good thermal stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1: the preparation of thermophilic high temperature resistant transaldolase
[0038] The preparation method of thermophilic and high temperature resistant transaldolase comprises strain, plasmid, enzyme and culture medium, PCR amplification and construction of recombinant plasmid, induced expression of gene and purification of protein. Specific steps are as follows:
[0039]The first step is to amplify the DNA fragment according to the upstream and downstream primers and use the whole genome sequence of Thermotoga maritima as a template. After the fragment is recovered, it is double-digested with NdeI and SalI endonucleases, and then connected to the same endonuclease The enzyme-digested expression plasmid vector pET28a was transformed into Escherichia coli E.coli DH5α, the recombinant plasmid was screened, and double-digested and sequenced for verification.
[0040] The upstream and downstream primers are respectively:
[0041] Upstream 5'-GGAATTC CATATG ...
Embodiment 2
[0048] Example 2: Basic enzymatic properties of transaldolase
[0049] The enzyme reaction equation is:
[0050] Erythrose-4-phosphate + Fructose-6-phosphate → Sedoheptulose-7-phosphate + Glyceraldehyde-3-phosphate
[0051] Enzyme reaction system: 500μl, 50mM phosphate buffer (pH 10) reaction system containing 0.6mM fructose-6-phosphate, 0.5mM erythrose-4-phosphate, 10μl purified enzyme, reaction temperature 80℃, reaction time 1min .
[0052] Through the analysis and identification of transaldolase, the main enzymatic properties of the enzyme are as follows:
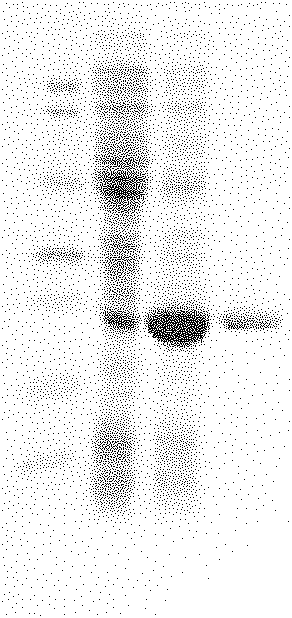
[0053] (1) The purified transaldolase protein electrophoresis pattern that obtains is as follows figure 1 shown.
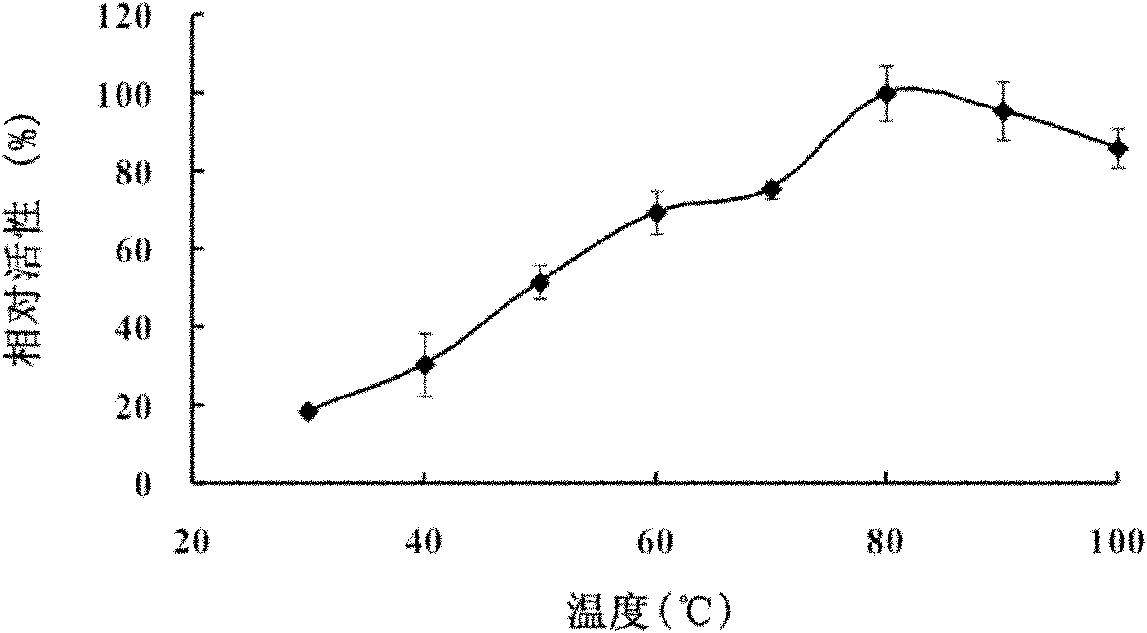
[0054] (2) The optimal temperature of the enzyme is 80°C (such as figure 2 shown)
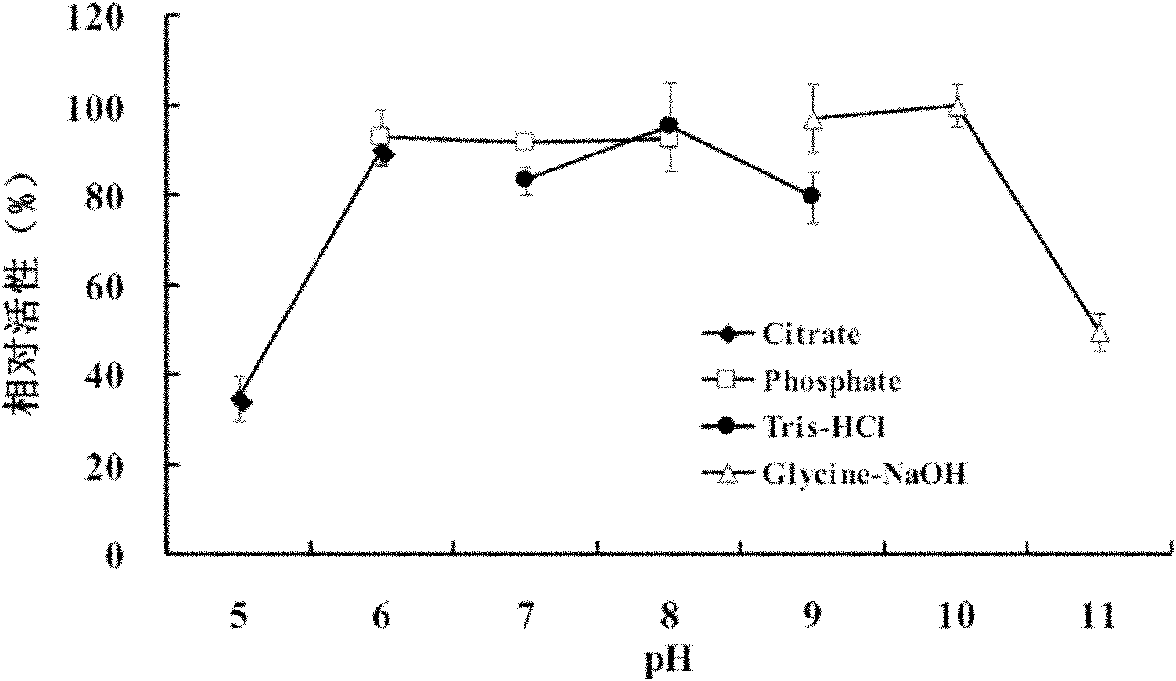
[0055] (3) The enzyme has a wider optimum pH, and has a high enzyme activity in the range of pH 6-10, and the optimum enzyme activity pH is 10.0 (such as image 3 shown)
[0056] (4) Under the experimental condition...
Embodiment 3
[0057] Embodiment 3: the thermostability analysis of transaldolase
[0058] Thermal stability analysis method: place the prepared transaldolase solution (1mg / ml) in water baths at 60°C and 80°C respectively, and measure it at 0, 0.5, 1, The remaining enzyme activity at 2, 4, 8, 12, 24, and 48 hours. The method for measuring enzyme activity is the same as in Example 3.
[0059] Through the thermostability analysis identification of this transaldolase, obtain following result:
[0060] The enzyme has high thermal stability, and the enzyme activity is 80% and 10% of the initial enzyme activity after being placed at 60°C and 80°C for 48 hours. At this point the activity is still as high as 33.53U / mg and 3.94U / mg.
[0061] Among them, 1 U / mg of enzyme activity represents 1 μmol of substrate that can be converted per minute per mg of protein.
[0062] In 2004, a thermophilic transaldolase was successfully cloned and expressed from the thermophilic bacterium Methanocaldococcus ja...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com