Gentamycin sulfate capsule and preparation method thereof
A technology of gentamicin sulfate and capsules, which is applied in the fields of capsule transportation, pharmaceutical formulations, medical preparations of non-active ingredients, etc. It can solve the problems of difficult filling of capsules and unstable quality, and achieve fast dissolution rate and good stability , fast disintegration effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
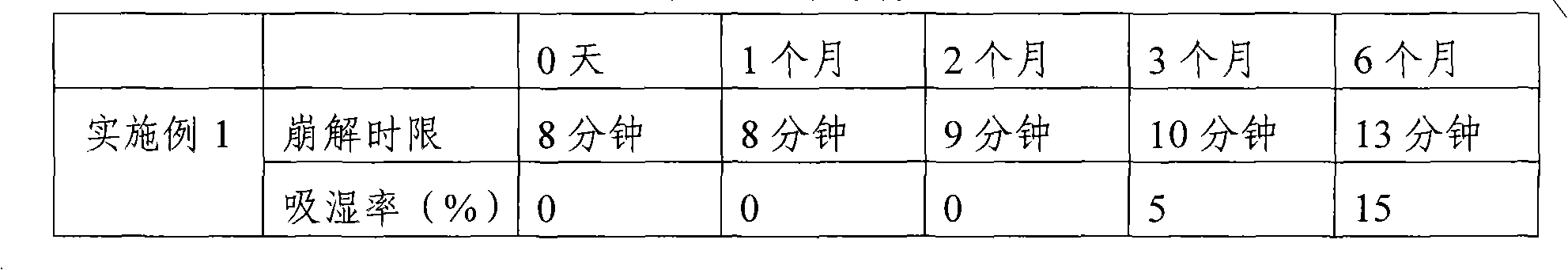
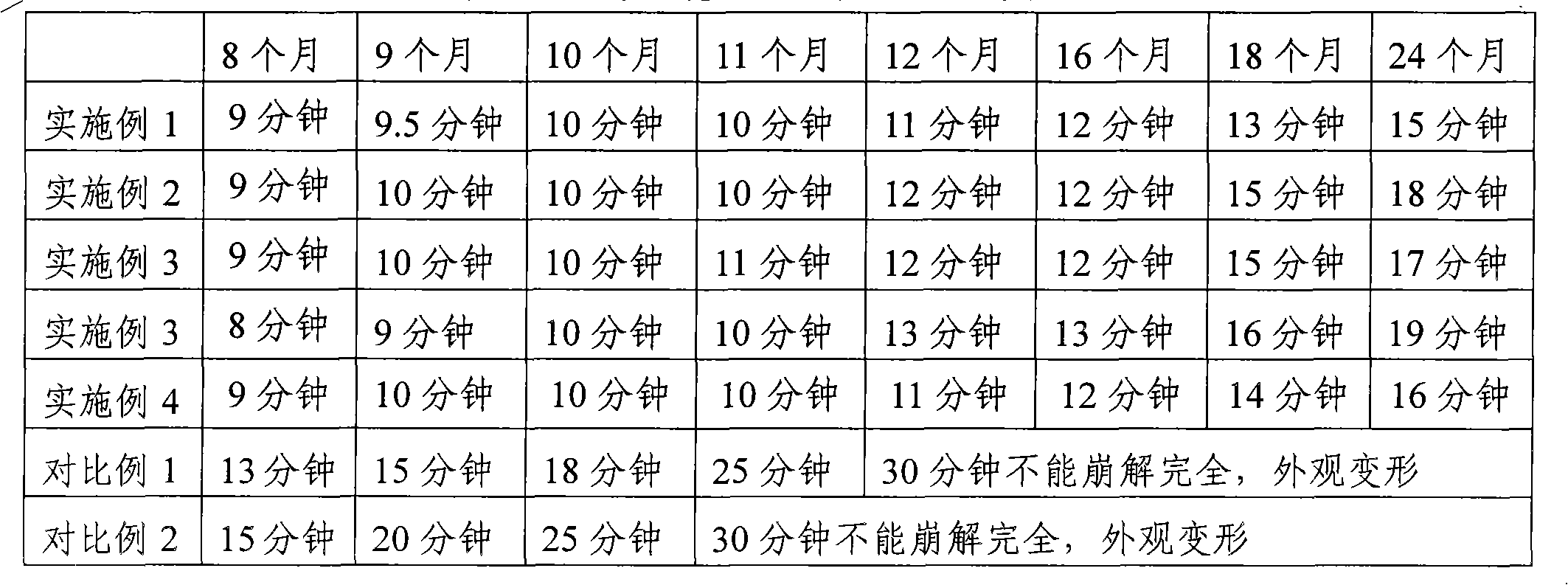
Embodiment 1
[0033] 1. Composition:
[0034] composition
Weight (g)
percentage(%)
750
30.61
700
28.57
microcrystalline methyl cellulose
200
8.16
Calcium sulfate
500
20.44
100
4.08
200
8.16
total
2450
100
[0035] 2. Preparation method:
[0036] 1) Weigh gentamicin sulfate, microcrystalline cellulose, starch, and calcium sulfate according to the prescription, then pulverize them respectively, dry them for 2 hours at a temperature of 55° C., and pass through a 100-mesh sieve;
[0037] 2) Mix gentamicin sulfate, microcrystalline methyl cellulose, starch, and calcium sulfate powder uniformly according to the method of equal addition, and the powder after mixing is white or off-white powder;
[0038] 3) Hypromellose is prepared into a concentration of 5% as an adhesive to make a soft material, ...
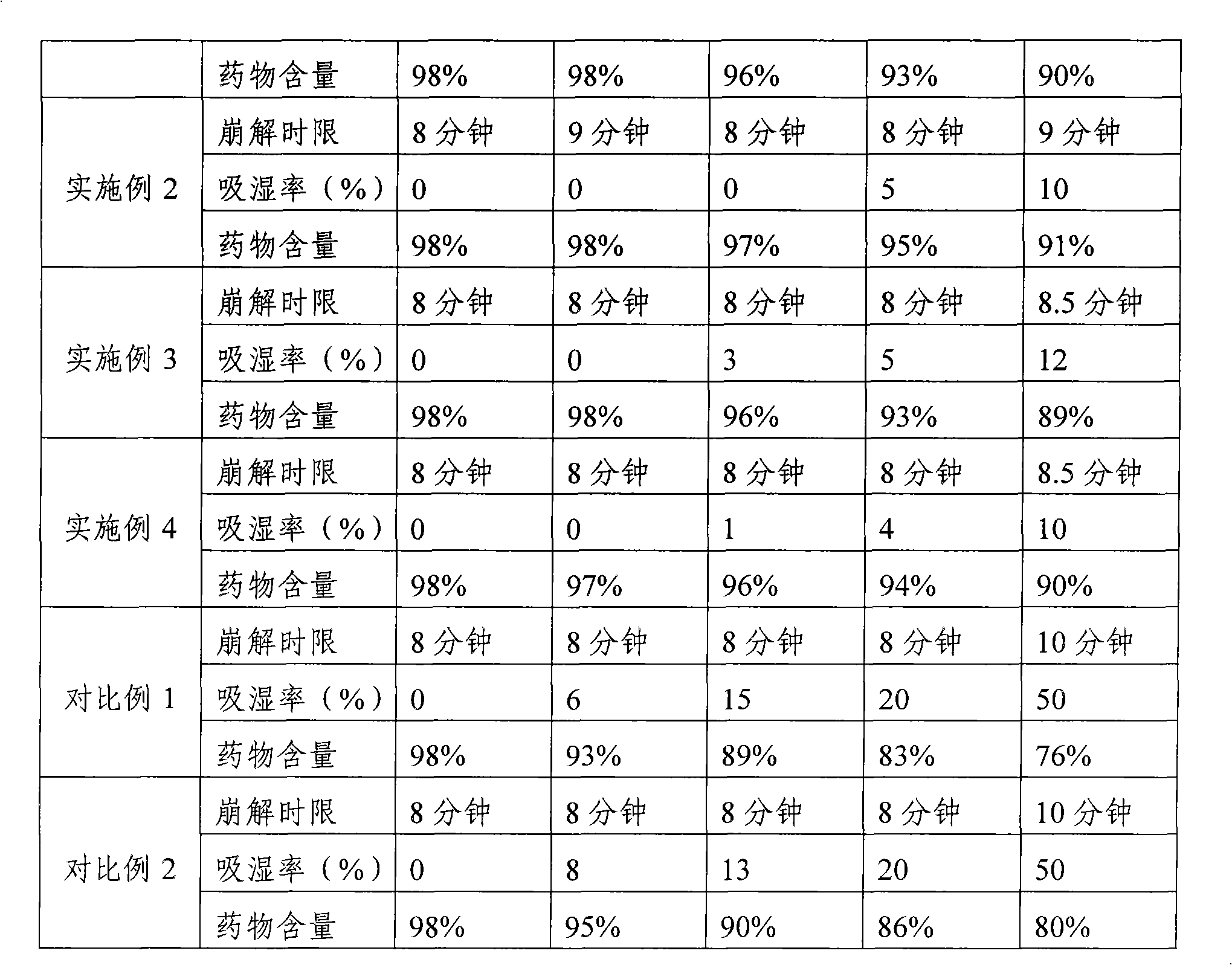
Embodiment 2
[0041] 1. Composition:
[0042] composition
Weight (g)
percentage(%)
750
27.27
1100
40
microcrystalline methyl cellulose
200
7.27
Calcium sulfate
400
14.55
100
3.64
[0043] silica
200
7.27
total
2750
100
[0044] 2. Preparation method:
[0045] 1) Weigh gentamicin sulfate, microcrystalline cellulose, pregelatinized starch, and calcium sulfate according to the composition, then pulverize them respectively, dry them for 2 hours at a temperature of 60° C., and pass through a 100-mesh sieve;
[0046] 2) Mix gentamicin sulfate, microcrystalline methyl cellulose, pregelatinized starch, and calcium sulfate powder evenly according to the method of equal addition, and the powder after mixing is white or off-white powder;
[0047] 3) Hypromellose is prepared into a concentration of...
Embodiment 3
[0050] 1. Composition:
[0051] composition
Weight (g)
percentage(%)
750
27.27
starch
1400
50.91
microcrystalline methyl cellulose
20
0.73
Calcium sulfate
200
7.27
Hypromellose
20
0.73
talcum powder
360
13.09
total
2750
100
[0052] 2. Preparation method:
[0053] 1) Weigh gentamicin sulfate, microcrystalline cellulose, starch, and calcium sulfate according to the composition, then pulverize them respectively, dry them at 60°C for 2 hours, and pass through a 100-mesh sieve;
[0054] 2) Mix the mixed powder of gentamicin sulfate and microcrystalline methyl cellulose with the mixed powder of starch and calcium sulfate according to the method of equal increase, and the mixed powder is white or off-white powder;
[0055] 3) Hypromellose is prepared into a concentration of 5% as an adhesive to make a soft material, and th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com