Thermostable lipase, expression of coding gene of thermostable lipase and applications of thermostable lipase
A technology of lipase and amino acid, which is applied in the field of genetic engineering and enzyme engineering, can solve the problems of non-wide range, enzyme instability, difficult application range, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0132] Cloning and obtaining of embodiment 1 bacillus subtilis lipase coding gene
[0133] Extraction of genomic DNA: cultured at 37°C for 2 days, the cell concentration OD 600nm Subtilis (Bacillus subtilis subsp.subtilis str.BGSC 1A700, (Bacillus Genetic Stock Center (Columbus, Ohio, USA))) 0.5-0.8 bacterium solution 50ml, centrifuged at 10000rpm for 10 minutes, take 50mg of bacteria and add 500μl sterile water Wash, and centrifuge to collect the precipitate. Resuspend the pellet in 500μl 1mg / ml lysozyme solution (Shanghai Sangong), incubate at 37°C for 30 minutes, then add 100μl lysozyme solution and continue to incubate at 40-50°C for 30 minutes until the bacterial solution is transparent, add 10% SDS to a final concentration of 2% (m / v), stirred for about 5 minutes until the viscosity of the bacterial liquid decreased significantly, and centrifuged at 15,000 rpm for 10 minutes to remove debris. The supernatant was sequentially extracted with equal volumes of phenol, phen...
Embodiment 2
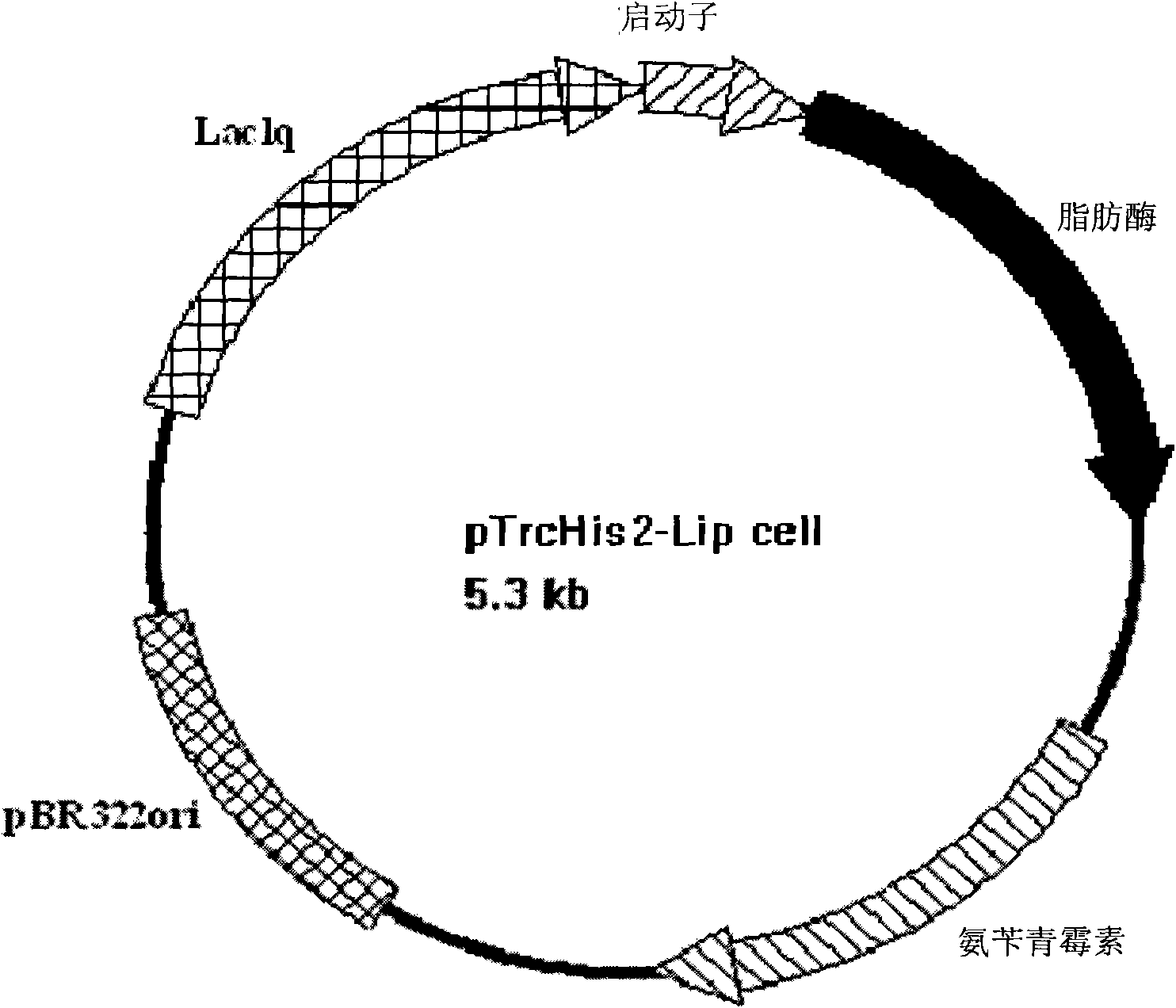
[0135] Embodiment 2 constructs Escherichia coli expression vector and expresses lipase in Escherichia coli
[0136] Design primers according to the sequence of pTrcHis2 plasmid
[0137] pTrcHis2 leader primer:
[0138] 5′-GTATATATTAATGTATCGATTAAATAAGGAGGAATAAA CTCGAG CCCTTAAGGGC-3' (SEQ ID NO: 3) and pTrcHis2 reverse primer: 5'-GAATTCGCCCTT
[0139] AAGGG CTCGAG TTTATTTCCTCCTTA-3' (SEQ ID NO: 4), introduce restriction site XhoI (underlined part), use pTrcHis2 plasmid as template, carry out PCR amplification, PCR reaction parameters: 95°C denaturation for 2 minutes; then 95°C denaturation 20 seconds, annealing at 60°C for 10 seconds, extension at 68°C for 2 minutes and 10 seconds, and after 18 cycles, hold at 68°C for 5 minutes. Collect the product, carry out agarose gel electrophoresis and recover the DNA fragment near 4400bp with the gel extraction kit (E.Z.N.A. gel extraction kit of OMEGA Company), obtain the pTrcHis2 plasmid with the new restriction site.
[0140] Ac...
Embodiment 3
[0146] The preparation of embodiment 3 recombinant lipase
[0147] Get the recombinant Escherichia coli strain TOP10 of the Bacillus subtilis-derived lipase recombinant plasmid positive clone prepared in Example 2, inoculate in a bottle of 50 ml LB culture solution (250 ml Erlenmeyer flask, containing 80 μg / ml Amp), and shake at 37 ° C at 250 rpm To OD600nm=0.3~0.5 (about 2-3 hours), then inoculate the seeds in 3L fermentation basic medium (10g / L peptone, 5g / L yeast powder, 1g / L NaCl, 6g / L Na 2 HPO 4 12H 2 O, 3g / L KH 2 PO 4 , 6g / L (NH 4 ) 2 SO 4 , 1g / L MgSO 4 ·7H 2 O, 0.01g / L CaCl 2 , 15g / L Glucose, 0.05g / L Amp, 0.1g / L FeSO 4 . ), fermented in a 5L fermenter.
[0148] In the initial stage---thalline growth stage, adjust the pH with 25% ammoniacal liquor (v / v) in the fermentation process, make it maintain on 7.0-7.2, and add trace element solution (3.5 mM copper sulfate, 0.06mM sodium iodide, 1.8mM manganese sulfate, 0.08mM sodium molybdate, 0.04mM boric acid, 0.5m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Vitality | aaaaa | aaaaa |
| Boiling point | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com