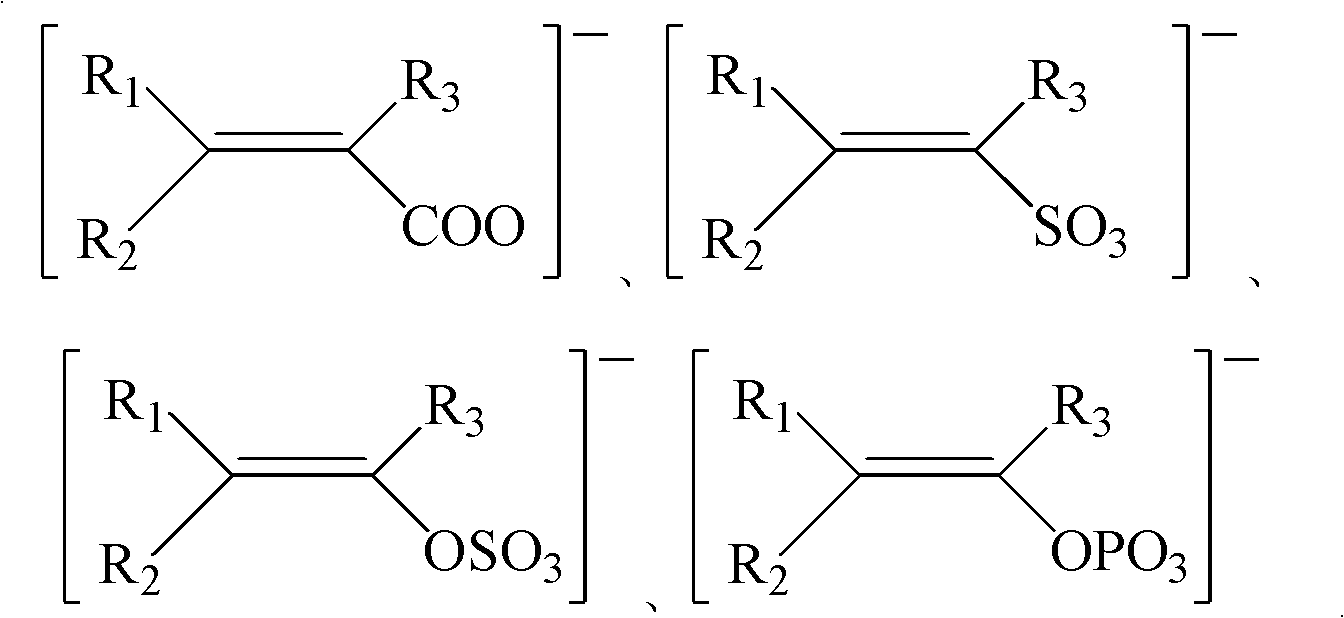
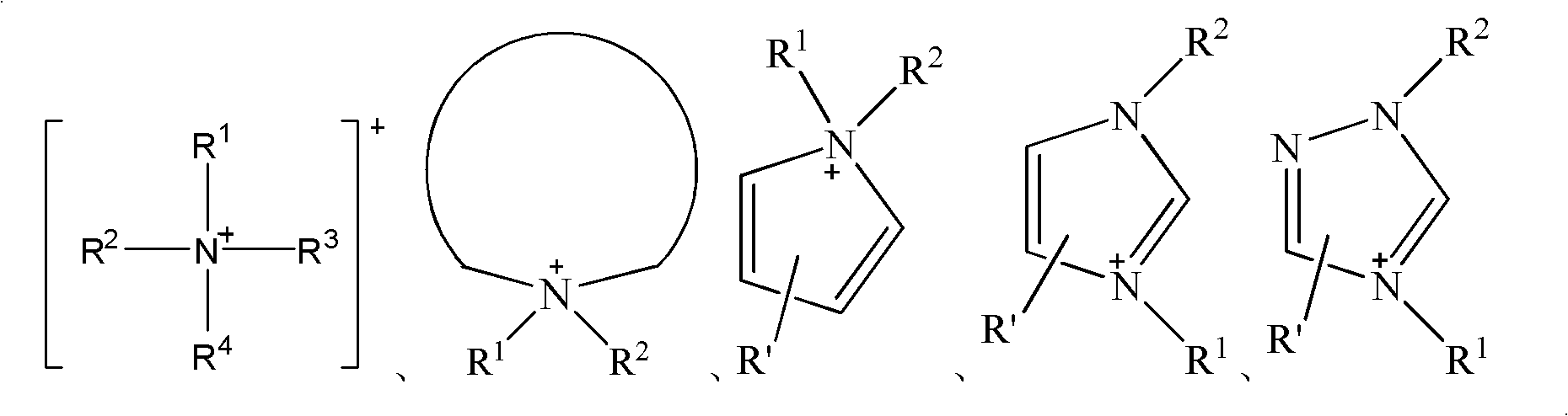
Ice-covering-proof coating containing electrolyte
An anti-icing and polyelectrolyte technology, applied in the field of materials, can solve the problems of ineffective prevention of icing and limited application effect of superhydrophobic materials, and achieve the effects of low cost, reduced adhesion, and anti-icing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Add 200mL of ethanol, 1g of vinyltrimethoxysilane, and 20g of acrylic acid into a 500mL three-necked flask, stir, and when the temperature rises to 70°C, add 0.15g of azobisisobutyronitrile, react for 5 hours, add 12g of NaOH into the flask, and continue stirring 3h, that is, silicone modified sodium acrylate resin.
[0031] In a 500mL three-neck flask, add 120g of water and 0.4g of sodium lauryl sulfate in sequence, and stir. Then 1.2g vinyltriethoxysilane, 15.0g dodecafluoroheptyl methacrylate, 10.0g butyl methacrylate, 0.3g benzoyl peroxide were mixed together, ultrasonicated for 10min, and then added to the three-necked bottle Medium, N 2 Protect, raise the temperature to 75°C, and react for 6 hours to obtain a colorless and transparent granular polymer, that is, a fluorosilicon-modified acrylic resin.
[0032] The coating formulation is shown in Table 1.
[0033] Table 1
[0034]
[0035]
[0036] Apply the prepared anti-icing coating to the steel core al...
Embodiment 2
[0040] In ensuring that other conditions remain unchanged in Example 1, vinyl trimethylethoxysilane is followed by vinyl triethoxysilane, propenyl trimethoxysilane, propenyl triethoxysilane, gamma-methacryloxy Propyltrimethoxysilane, γ-methacryloxypropyltrimethoxysilane or methacryloxypropyltri(isopropoxy)silane, trifluoropropyltrimethoxysilane, trifluoropropyl Methyldichlorosilane, Trifluoropropylmethylcyclotrisiloxane, Tridecafluorooctyltrimethoxysilane, Trifluoromethyltrimethylsilane, 1H, 1H, 2H, 2H-Perfluorooctyltrimethylsilane (B) oxysilane is replaced to obtain the corresponding organosilicon-modified sodium polyacrylate resin.
[0041] In a 500mL three-neck flask, add 120g of water and 0.4g of sodium lauryl sulfate in sequence, and stir.
[0042] Then 1.2g vinyltriethoxysilane, 15.0g dodecafluoroheptyl methacrylate, 10.0g butyl methacrylate, 0.3g benzoyl peroxide were mixed together, ultrasonicated for 10min, and then added to the three-necked bottle Medium, N 2 Prot...
Embodiment 3
[0047] Add 200mL ethanol, 1.0g vinyltrimethoxysilane, 0.1g trifluoropropylmethyldichlorosilane, 22g acrylic acid into a 500mL three-necked flask, stir, and when the temperature rises to 75°C, add 0.18g azobisisobutyronitrile , reacted for 5 hours, added 13.6g NaOH to the flask, and continued to stir for 3h to obtain silicone-modified sodium acrylate resin.
[0048] In a 500mL three-neck flask, add 120g of water and 0.4g of sodium lauryl sulfate in sequence, and stir. Then 1.2g vinyltriethoxysilane, 15.0g dodecafluoroheptyl methacrylate, 10.0g butyl methacrylate, 0.3g benzoyl peroxide were mixed together, ultrasonicated for 10min, and then added to the three-necked bottle Medium, N 2 Protect, raise the temperature to 75°C, and react for 6 hours to obtain a colorless and transparent granular polymer, that is, a fluorosilicon-modified acrylic resin.
[0049] The paint formulations are shown in Table 4.
[0050] Table 4
[0051]
[0052] *The size of nano-silica sol is betw...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com